In Vitro Mode of Action and Anti-thrombotic Activity of Boophilin, a Multifunctional Kunitz Protease Inhibitor from the Midgut of a Tick Vector of Babesiosis, Rhipicephalus microplus
- PMID: 26745503
- PMCID: PMC4706430
- DOI: 10.1371/journal.pntd.0004298
In Vitro Mode of Action and Anti-thrombotic Activity of Boophilin, a Multifunctional Kunitz Protease Inhibitor from the Midgut of a Tick Vector of Babesiosis, Rhipicephalus microplus
Abstract
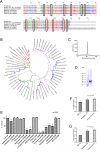
Background: Hematophagous mosquitos and ticks avoid host hemostatic system through expression of enzyme inhibitors targeting proteolytic reactions of the coagulation and complement cascades. While most inhibitors characterized to date were found in the salivary glands, relatively few others have been identified in the midgut. Among those, Boophilin is a 2-Kunitz multifunctional inhibitor targeting thrombin, elastase, and kallikrein. However, the kinetics of Boophilin interaction with these enzymes, how it modulates platelet function, and whether it inhibits thrombosis in vivo have not been determined.
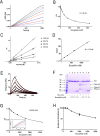
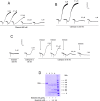
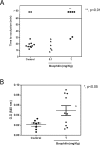
Methodology/principal findings: Boophilin was expressed in HEK293 cells and purified to homogeneity. Using amidolytic assays and surface plasmon resonance experiments, we have demonstrated that Boophilin behaves as a classical, non-competitive inhibitor of thrombin with respect to small chromogenic substrates by a mechanism dependent on both exosite-1 and catalytic site. Inhibition is accompanied by blockade of platelet aggregation, fibrin formation, and clot-bound thrombin in vitro. Notably, we also identified Boophilin as a non-competitive inhibitor of FXIa, preventing FIX activation. In addition, Boophilin inhibits kallikrein activity and the reciprocal activation, indicating that it targets the contact pathway. Furthermore, Boophilin abrogates cathepsin G- and plasmin-induced platelet aggregation and partially affects elastase-mediated cleavage of Tissue Factor Pathway Inhibitor (TFPI). Finally, Boophilin inhibits carotid artery occlusion in vivo triggered by FeCl3, and promotes bleeding according to the mice tail transection method.
Conclusion/significance: Through inhibition of several enzymes involved in proteolytic cascades and cell activation, Boophilin plays a major role in keeping the midgut microenvironment at low hemostatic and inflammatory tonus. This response allows ticks to successfully digest a blood meal which is critical for metabolism and egg development. Boophilin is the first tick midgut FXIa anticoagulant also found to inhibit thrombosis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Louw E, van der Merwe NA, Neitz AW, Maritz-Olivier C (2012) Evolution of the tissue factor pathway inhibitor-like Kunitz domain-containing protein family in Rhipicephalus microplus. Int J Parasitol. - PubMed
-
- Ribeiro JM, Francischetti IM (2003) Role of arthropod saliva in blood feeding: sialome and post-sialome perspectives. Annu Rev Entomol 48: 73–88. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

