Intrauterine Growth Restriction Alters Mouse Intestinal Architecture during Development
- PMID: 26745886
- PMCID: PMC4706418
- DOI: 10.1371/journal.pone.0146542
Intrauterine Growth Restriction Alters Mouse Intestinal Architecture during Development
Abstract
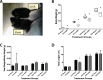
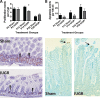
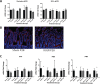
Infants with intrauterine growth restriction (IUGR) are at increased risk for neonatal and lifelong morbidities affecting multiple organ systems including the intestinal tract. The underlying mechanisms for the risk to the intestine remain poorly understood. In this study, we tested the hypothesis that IUGR affects the development of goblet and Paneth cell lineages, thus compromising the innate immunity and barrier functions of the epithelium. Using a mouse model of maternal thromboxane A2-analog infusion to elicit maternal hypertension and resultant IUGR, we tested whether IUGR alters ileal maturation and specifically disrupts mucus-producing goblet and antimicrobial-secreting Paneth cell development. We measured body weights, ileal weights and ileal lengths from birth to postnatal day (P) 56. We also determined the abundance of goblet and Paneth cells and their mRNA products, localization of cellular tight junctions, cell proliferation, and apoptosis to interrogate cellular homeostasis. Comparison of the murine findings with human IUGR ileum allowed us to verify observed changes in the mouse were relevant to clinical IUGR. At P14 IUGR mice had decreased ileal lengths, fewer goblet and Paneth cells, reductions in Paneth cell specific mRNAs, and decreased cell proliferation. These findings positively correlated with severity of IUGR. Furthermore, the decrease in murine Paneth cells was also seen in human IUGR ileum. IUGR disrupts the normal trajectory of ileal development, particularly affecting the composition and secretory products of the epithelial surface of the intestine. We speculate that this abnormal intestinal development may constitute an inherent "first hit", rendering IUGR intestine susceptible to further injury, infection, or inflammation.
Conflict of interest statement
Figures





Similar articles
-
Fibroblast growth factor 10 alters the balance between goblet and Paneth cells in the adult mouse small intestine.Am J Physiol Gastrointest Liver Physiol. 2015 Apr 15;308(8):G678-90. doi: 10.1152/ajpgi.00158.2014. Epub 2015 Feb 26. Am J Physiol Gastrointest Liver Physiol. 2015. PMID: 25721301 Free PMC article.
-
Postnatal nutritional restriction affects growth and immune function of piglets with intra-uterine growth restriction.Br J Nutr. 2015 Jul 14;114(1):53-62. doi: 10.1017/S0007114515001579. Epub 2015 Jun 10. Br J Nutr. 2015. PMID: 26059215
-
Intrauterine growth retardation promotes fetal intestinal autophagy in rats via the mechanistic target of rapamycin pathway.J Reprod Dev. 2017 Dec 15;63(6):547-554. doi: 10.1262/jrd.2017-050. Epub 2017 Aug 31. J Reprod Dev. 2017. PMID: 28855439 Free PMC article.
-
The Paneth Cell: The Curator and Defender of the Immature Small Intestine.Front Immunol. 2020 Apr 3;11:587. doi: 10.3389/fimmu.2020.00587. eCollection 2020. Front Immunol. 2020. PMID: 32308658 Free PMC article. Review.
-
Dysbiosis--a consequence of Paneth cell dysfunction.Semin Immunol. 2013 Nov 30;25(5):334-41. doi: 10.1016/j.smim.2013.09.006. Epub 2013 Nov 14. Semin Immunol. 2013. PMID: 24239045 Review.
Cited by
-
Loss of murine Paneth cell function alters the immature intestinal microbiome and mimics changes seen in neonatal necrotizing enterocolitis.PLoS One. 2018 Oct 1;13(10):e0204967. doi: 10.1371/journal.pone.0204967. eCollection 2018. PLoS One. 2018. PMID: 30273395 Free PMC article.
-
Intrauterine Growth Restriction Causes Abnormal Embryonic Dentate Gyrus Neurogenesis in Mouse Offspring That Leads to Adult Learning and Memory Deficits.eNeuro. 2021 Oct 8;8(5):ENEURO.0062-21.2021. doi: 10.1523/ENEURO.0062-21.2021. Print 2021 Sep-Oct. eNeuro. 2021. PMID: 34544755 Free PMC article.
-
Effects of dietary Bacillus amyloliquefaciens supplementation on growth performance, intestinal morphology, inflammatory response, and microbiota of intra-uterine growth retarded weanling piglets.J Anim Sci Biotechnol. 2018 Mar 13;9:22. doi: 10.1186/s40104-018-0236-2. eCollection 2018. J Anim Sci Biotechnol. 2018. PMID: 29564121 Free PMC article.
-
Adaptive Physiological and Morphological Adjustments Mediated by Intestinal Stem Cells in Response to Food Availability in Mice.Front Physiol. 2019 Jan 8;9:1821. doi: 10.3389/fphys.2018.01821. eCollection 2018. Front Physiol. 2019. PMID: 30670976 Free PMC article.
-
Intrauterine growth restriction and its impact on intestinal morphophysiology throughout postnatal development in pigs.Sci Rep. 2022 Jul 12;12(1):11810. doi: 10.1038/s41598-022-14683-z. Sci Rep. 2022. PMID: 35821501 Free PMC article.
References
-
- Baserga M, Bertolotto C, Maclennan NK, Hsu JL, Pham T, Laksana GS, et al. Uteroplacental insufficiency decreases small intestine growth and alters apoptotic homeostasis in term intrauterine growth retarded rats. Early human development. 2004;79(2):93–105. 10.1016/j.earlhumdev.2004.04.015 . - DOI - PubMed
-
- Bernstein IM, Horbar JD, Badger GJ, Ohlsson A, Golan A. Morbidity and mortality among very-low-birth-weight neonates with intrauterine growth restriction. The Vermont Oxford Network. American journal of obstetrics and gynecology. 2000;182(1 Pt 1):198–206. . - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

