Patients' willingness to participate in clinical trials and their views on aspects of cancer research: results of a prospective patient survey
- PMID: 26745891
- PMCID: PMC4706669
- DOI: 10.1186/s13063-015-1105-3
Patients' willingness to participate in clinical trials and their views on aspects of cancer research: results of a prospective patient survey
Abstract
Background: Recruitment to clinical trials can be challenging and slower than anticipated. This prospective patient survey aimed to investigate the proportion of patients approached about a trial who agree to participate, their motivations for trial participation and their views on aspects of cancer research.
Methods: Patients who had been approached about participation in any clinical trials in the Gastrointestinal and Lymphoma Unit at the Royal Marsden were invited to complete a questionnaire. The statistical analysis is mainly descriptive, with percentages being reported. Univariate logistic regression analysis was used to determine any associations between patient characteristics and patient responses.
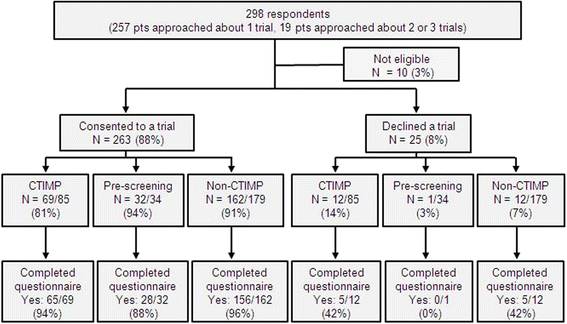
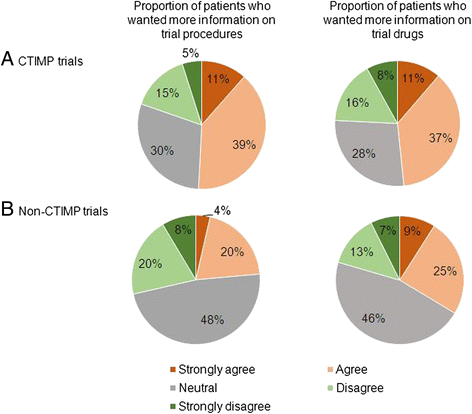
Results: From August 2013-July 2014, 276 patients received 298 clinical trial patient information sheets and were asked to complete the questionnaire. The majority of patients (263 patients, 88 %) consented to a clinical trial and 249 of the 263 patients (95 %) completed the questionnaire. Multiple factors influenced decisions to participate in clinical trials, with patients stating that the most important reasons were that the trial offered the best treatment available and that the trial results could benefit others. Of the 249 questionnaire respondents, 78 % would donate their tissue for genetic research, 75 % would consider participating in studies requiring a research biopsy and 75 % felt that patients should be informed of trial results. Patients treated with palliative intent and those who had received multiple lines of treatment were more willing to consider research biopsies. Of the patients approached about a clinical trial of an investigational medicinal product, 48-50 % would have liked more information on the study drugs/procedures.
Conclusion: The majority of patients approached about a clinical trial consented to one or more trials. Patients' motivations for trial participation included potential personal benefit and altruistic reasons. A high proportion of patients were willing to donate tissue for research and to consider trials involving repeat biopsies. The majority of patients feel that participants should be informed of trial results and there is a group of patients who would like more detailed trial information.
Figures



References
-
- Stensland K, McBride R, Wisnivesky J, Asma Latif A, Hendricks R, Roper N, et al. Premature termination of genitourinary cancer clinical trials. J Clin Oncol. 2014;32(Suppl 4):abstr 288).
-
- National Institute for Health Research. Performance in Initiating and Delivering Research. 2015 http://www.nihr.ac.uk/policy-and-standards/Performance-in-initiating-and.... Accessed on 08.01.16.
-
- Cancer Research UK. Our research on clinical trials and new treatments. 2014. http://www.cancerresearchuk.org/our-research/our-research-by-cancersubje.... Accessed on 08.01.16.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

