Clinical usefulness of gene-expression profile to rule out acute rejection after heart transplantation: CARGO II
- PMID: 26746629
- PMCID: PMC5015661
- DOI: 10.1093/eurheartj/ehv682
Clinical usefulness of gene-expression profile to rule out acute rejection after heart transplantation: CARGO II
Abstract
Aims: A non-invasive gene-expression profiling (GEP) test for rejection surveillance of heart transplant recipients originated in the USA. A European-based study, Cardiac Allograft Rejection Gene Expression Observational II Study (CARGO II), was conducted to further clinically validate the GEP test performance.
Methods and results: Blood samples for GEP testing (AlloMap(®), CareDx, Brisbane, CA, USA) were collected during post-transplant surveillance. The reference standard for rejection status was based on histopathology grading of tissue from endomyocardial biopsy. The area under the receiver operating characteristic curve (AUC-ROC), negative (NPVs), and positive predictive values (PPVs) for the GEP scores (range 0-39) were computed. Considering the GEP score of 34 as a cut-off (>6 months post-transplantation), 95.5% (381/399) of GEP tests were true negatives, 4.5% (18/399) were false negatives, 10.2% (6/59) were true positives, and 89.8% (53/59) were false positives. Based on 938 paired biopsies, the GEP test score AUC-ROC for distinguishing ≥3A rejection was 0.70 and 0.69 for ≥2-6 and >6 months post-transplantation, respectively. Depending on the chosen threshold score, the NPV and PPV range from 98.1 to 100% and 2.0 to 4.7%, respectively.
Conclusion: For ≥2-6 and >6 months post-transplantation, CARGO II GEP score performance (AUC-ROC = 0.70 and 0.69) is similar to the CARGO study results (AUC-ROC = 0.71 and 0.67). The low prevalence of ACR contributes to the high NPV and limited PPV of GEP testing. The choice of threshold score for practical use of GEP testing should consider overall clinical assessment of the patient's baseline risk for rejection.
Trial registration: ClinicalTrials.gov NCT00761787.
Keywords: AlloMap; Heart transplant; Molecular diagnostics; Organ rejection; Rejection surveillance; Tests.
© The Author 2016. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures
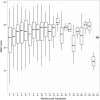
References
-
- Deng MC, Eisen HJ, Mehra MR, Billingham M, Marboe CC, Berry G, Kobashigawa J, Johnson FL, Starling RC, Murali S, Pauly DF, Baron H, Wohlgemuth JG, Woodward RN, Klingler TM, Walther D, Lal PG, Rosenberg S, Hunt S, CARGO Investigators. Noninvasive discrimination of rejection in cardiac allograft recipients using gene expression profiling. Am J Transplant 2006;6:150–160. - PubMed
-
- Starling RC, Pham M, Valantine H, Miller L, Eisen H, Rodriguez ER, Taylor DO, Yamani MH, Kobashigawa J, McCurry K, Marboe C, Mehra MR, Zuckerman A, Deng MC, Working Group on Molecular Testing in Cardiac Transplantation. Molecular testing in the management of cardiac transplant recipients: initial clinical experience. J Heart Lung Transplant 2006;25:1389–1395. - PubMed
-
- Pham MX, Teuteberg JJ, Kfoury AG, Starling RC, Deng MC, Cappola TP, Kao A, Anderson AS, Cotts WG, Ewald GA, Baran DA, Bogaev RC, Elashoff B, Baron H, Yee J, Valantine HA, IMAGE Study Group. Gene expression profiling for rejection surveillance after cardiac transplantation. N Engl J Med 2010;362:1890–1900. - PubMed
-
- Costanzo MR, Dipchand A, Starling R, Anderson A, Chan M, Desai S, Fedson S, Fisher P, Gonzales-Stawinski G, Martinelli L, McGiffin D, Smith J, Taylor D, Meiser B, Webber S, Baran D, Carboni M, Dengler T, Feldman D, Frigerio M, Kfoury A, Kim D, Kobashigawa J, Shullo M, Stehlik J, Teuteberg J, Uber P, Zuckermann A, Hunt S, Burch M, Bhat G, Canter C, Chinnock R, Crespo-Leiro M, Delgado R, Dobbels F, Grady K, Kao W, Lamour J, Parry G, Patel J, Pini D, Towbin J, Wolfel G, Delgado D, Eisen H, Goldberg L, Hosenpud J, Johnson M, Keogh A, Lewis C, O'Connell J, Rogers J, Ross H, Russell S, Vanhaecke J, International Society of Heart and Lung Transplantation Guidelines. The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients. J Heart Lung Transplant 2010;29:914–956. - PubMed
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

