Cells of renin lineage express hypoxia inducible factor 2α following experimental ureteral obstruction
- PMID: 26746687
- PMCID: PMC4706659
- DOI: 10.1186/s12882-015-0216-0
Cells of renin lineage express hypoxia inducible factor 2α following experimental ureteral obstruction
Abstract
Background: Recent studies indicate that mural cells of the preglomerular vessels, known as cells of renin lineage (CoRL), contribute to repair and regeneration of injured kidney glomeruli. However, their potential roles in tubulointerstitial disease are less understood. The aim of this study was to better understand CoRL number and distribution following UUO so that future mechanistic studies could be undertaken.
Methods: We mapped the fate of CoRL in adult Ren1cCreER x Rs-tdTomato-R reporter mice that underwent UUO. Kidney biopsies from sham and UUO-subjected mice on days 3, 7, and 14 were evaluated by immunohistochemistry.
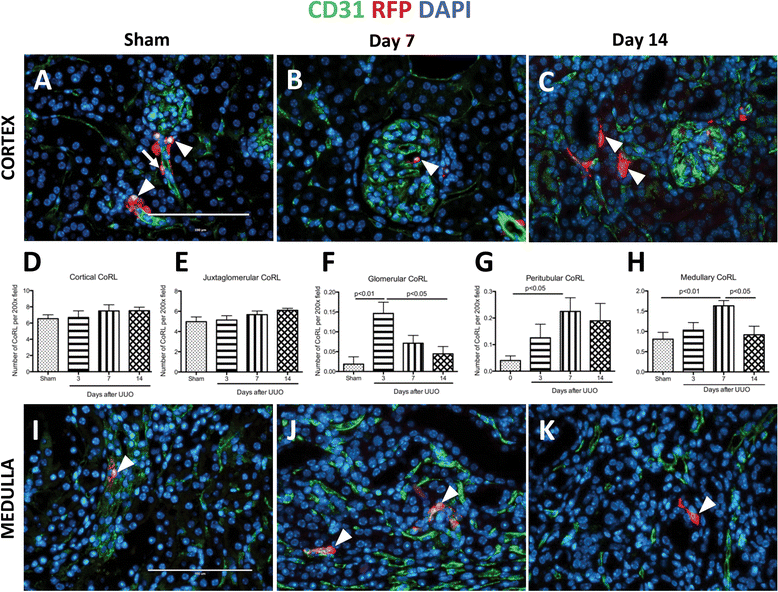
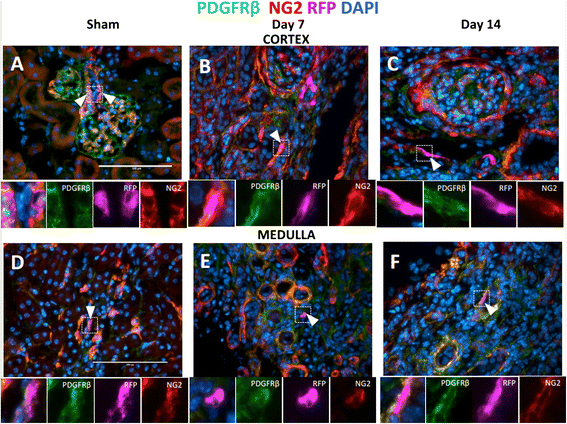
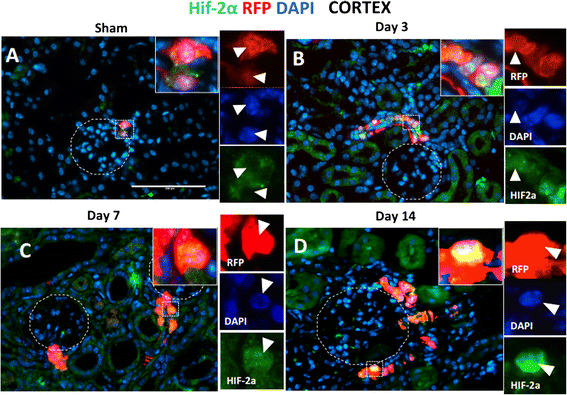
Results: In sham animals, CoRL were restricted to juxtaglomerular location. At day 7 following UUO, CoRL increased two-fold, were perivascular in location, and co-expressed pericyte markers (PDGFßR, NG2), but did not express renin. At day 14 post UUO, labeled CoRL detached from vessels and were present in the interstitium, in areas of fibrosis, where they now expressed the myofibroblast marker alpha-smooth muscle actin. The increase in CoRL was likely due to proliferation as marked by BrdU labeling, and migration from the cortex. Following UUO starting from day 3, active hypoxia inducible factor-2α was detected in nuclei in labeled CoRL, in the cortex, but not those cells found in medulla.
Conclusions: We have demonstrated that arteriolar CoRL are potential kidney progenitors that may contribute to the initial vascular regeneration. However, in chronic kidney injury (≥14 days post UUO), perivascular CoRL transition to myofibroblast-like cells.
Figures


References
-
- Moody TE, Vaughn ED, Gillenwater JY. Relationship between renal blood flow and ureteral pressure during 18 hours of total unilateral uretheral occlusion. Implications for changing sites of increased renal resistance. Invest Urol. 1975;13:246–51. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

