Neuronal HIF-1α and HIF-2α deficiency improves neuronal survival and sensorimotor function in the early acute phase after ischemic stroke
- PMID: 26746864
- PMCID: PMC5363746
- DOI: 10.1177/0271678X15624933
Neuronal HIF-1α and HIF-2α deficiency improves neuronal survival and sensorimotor function in the early acute phase after ischemic stroke
Abstract
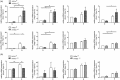
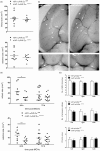
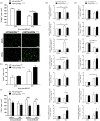
Hypoxia-inducible factors mediate adaptive responses to ischemia, among others, by induction of anti- and pro-survival genes. Thus, the impact of HIF on neuronal survival upon stroke is controversial. Therefore, neuron-specific knockout mice deficient for Hif1a and Hif2a were exposed to inspiratory hypoxia or ischemia-reperfusion injury. Both Hif1a- and Hif2a-deficient mice showed no altered infarct and edema size, suggesting that both HIF-α subunits might compensate for each other. Accordingly, hypoxic HIF-target gene regulation was marginally affected with exception of anti-survival Bnip3 and pro-survival erythropoietin. In the early acute stage upon stroke, Hif1a/Hif2a double knockout mice exhibited significantly reduced expression of the anti-survival Bnip3, Bnip3L, and Pmaip1 Accordingly, global cell death and edema were significantly reduced upon 24 h but not 72 h reperfusion. Behavioral assessment indicated that Hif1a/Hif2a-deficient mice initially performed better, but became significantly more impaired after 72 h accompanied by increased apoptosis and reduced angiogenesis. Our findings suggest that in neurons HIF-1 and HIF-2 have redundant functions for cellular survival under ischemic conditions. By contrast, lack of anti-survival factors in Hif1a/Hif2a-deficient mice might protect from early acute neuronal cell death and neurological impairment, indicating a benefit of HIF-pathway inhibition in neurons in the very acute phase after ischemic stroke.
Keywords: Erythropoietin; hypoxia-inducible factor; ischemia; stroke; vascular endothelial growth factor.
© The Author(s) 2016.
Figures




References
-
- Kaelin WG, Jr., Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell 2008; 30: 393–402. - PubMed
-
- Althaus J, Bernaudin M, Petit E, et al. Expression of the gene encoding the pro-apoptotic BNIP3 protein and stimulation of hypoxia-inducible factor-1alpha (HIF-1alpha) protein following focal cerebral ischemia in rats. Neurochem Int 2006; 48: 687–695. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

