Persistent Macular Thickening After Ranibizumab Treatment for Diabetic Macular Edema With Vision Impairment
- PMID: 26746868
- PMCID: PMC4786449
- DOI: 10.1001/jamaophthalmol.2015.5346
Persistent Macular Thickening After Ranibizumab Treatment for Diabetic Macular Edema With Vision Impairment
Abstract
Importance: The prevalence of persistent diabetic macular edema (DME) after months of anti-vascular endothelial growth factor therapy and its effect on visual acuity are unknown.
Objective: To assess subsequent outcomes of eyes with DME persisting for 24 weeks after initiating treatment with 0.5 mg of ranibizumab.
Design, setting, and participants: We performed post hoc, exploratory analyses of a randomized clinical trial from March 20, 2007, through January 29, 2014, from 117 of 296 eyes (39.5%) randomly assigned to receive ranibizumab with persistent DME (central subfield thickness ≥250 μm on time domain optical coherence tomography) through the 24-week visit.
Interventions: Four monthly intravitreous injections of ranibizumab and then as needed per protocol.
Main outcomes and measures: Cumulative 3-year probabilities of chronic persistent DME (failure to achieve a central subfield thickness <250 μm and at least a 10% reduction from the 24-week visit on at least 2 consecutive study visits) determined by life-table analyses, and at least 10 letter (≥2 line) gain or loss of visual acuity among those eyes.
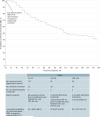
Results: The probability of chronic persistent DME among eyes with persistent DME at the 24-week visit decreased from 100% at the 32-week visit to 81.1% (99% CI, 69.6%-88.6%), 55.8% (99% CI, 42.9%-66.9%), and 40.1% (99% CI, 27.4%-52.4%) at the 1-, 2-, and 3-year visits, respectively. At 3 years, visual acuity improved in eyes with and without chronic persistent DME through the follow-up period, respectively, by a mean of 7 letters and 13 letters from baseline. Among 40 eyes with chronic persistent edema through 3 years, 17 (42.5%) (99% CI, 23.1%-63.7%) gained 10 letters or more from baseline, whereas 5 (12.5%) (99% CI, 2.8%-31.5%) lost 10 letters or more from baseline.
Conclusions and relevance: These data suggest less than half of eyes treated for DME with intravitreous ranibizumab have persistent central-involved DME through 24 weeks after initiating treatment. Among the 40% that then have chronic persistent central-involved DME through 3 years, longer-term visual acuity outcomes appear to be slightly worse than in the 60% in which DME does not persist. Nevertheless, when following the treatment protocol used in this trial among eyes with vision impairment from DME, long-term improvement in visual acuity from baseline is typical and substantial (≥2-line) loss of visual acuity is likely uncommon through 3 years, even when central-involved DME chronically persists.
Figures
Comment in
-
What Is Chronic or Persistent Diabetic Macular Edema and How Should It Be Treated?JAMA Ophthalmol. 2016 Mar;134(3):285-6. doi: 10.1001/jamaophthalmol.2015.5469. JAMA Ophthalmol. 2016. PMID: 26746003 No abstract available.
References
-
- Nguyen QD, Brown DM, Marcus DM, et al. RISE and RIDE Research Group. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119(4):789–801. - PubMed
-
- Mitchell P, Bandello F, Schmidt-Erfurth U, et al. RESTORE study group. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118(4):615–625. - PubMed
-
- Korobelnik JF, Do DV, Schmidt-Erfurth U, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014;121(11):2247–2254. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

