Increased peri-ductal collagen micro-organization may contribute to raised mammographic density
- PMID: 26747277
- PMCID: PMC4706673
- DOI: 10.1186/s13058-015-0664-2
Increased peri-ductal collagen micro-organization may contribute to raised mammographic density
Abstract
Background: High mammographic density is a therapeutically modifiable risk factor for breast cancer. Although mammographic density is correlated with the relative abundance of collagen-rich fibroglandular tissue, the causative mechanisms, associated structural remodelling and mechanical consequences remain poorly defined. In this study we have developed a new collaborative bedside-to-bench workflow to determine the relationship between mammographic density, collagen abundance and alignment, tissue stiffness and the expression of extracellular matrix organising proteins.
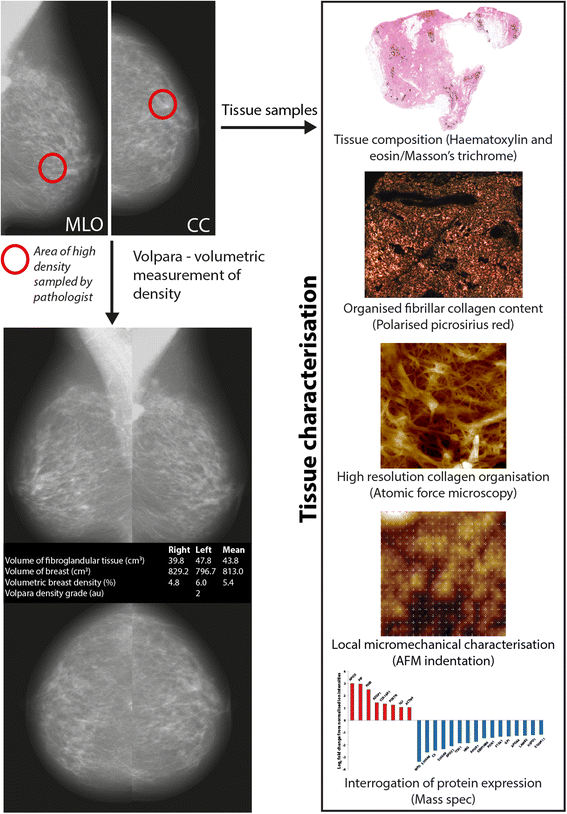
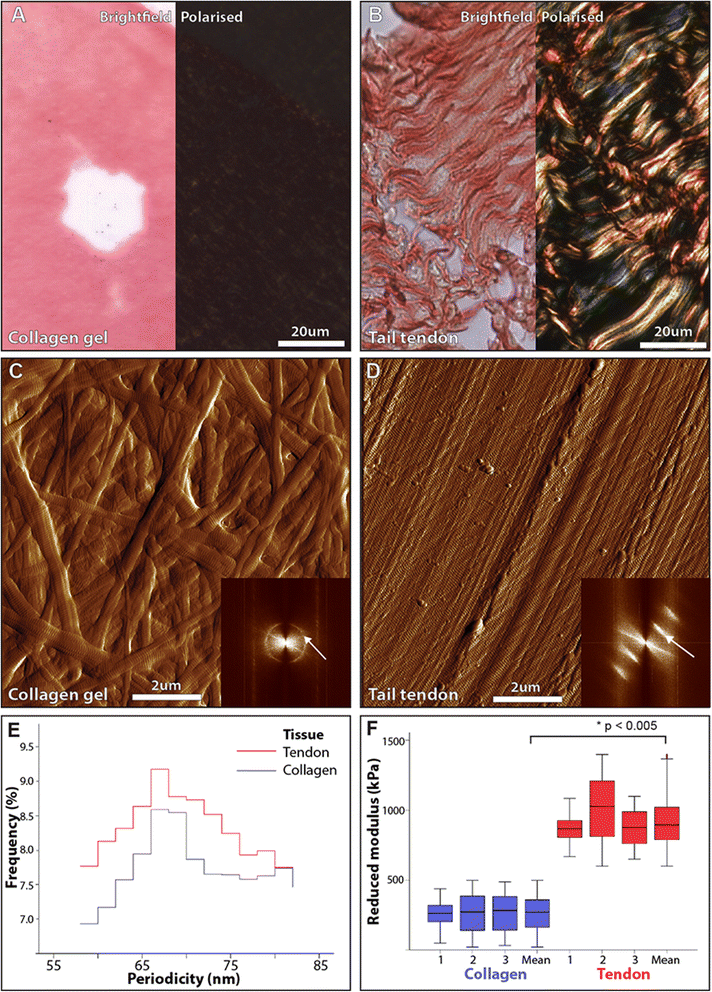
Methods: Mammographic density was assessed in 22 post-menopausal women (aged 54-66 y). A radiologist and a pathologist identified and excised regions of elevated non-cancerous X-ray density prior to laboratory characterization. Collagen abundance was determined by both Masson's trichrome and Picrosirius red staining (which enhances collagen birefringence when viewed under polarised light). The structural specificity of these collagen visualisation methods was determined by comparing the relative birefringence and ultrastructure (visualised by atomic force microscopy) of unaligned collagen I fibrils in reconstituted gels with the highly aligned collagen fibrils in rat tail tendon. Localised collagen fibril organisation and stiffness was also evaluated in tissue sections by atomic force microscopy/spectroscopy and the abundance of key extracellular proteins was assessed using mass spectrometry.
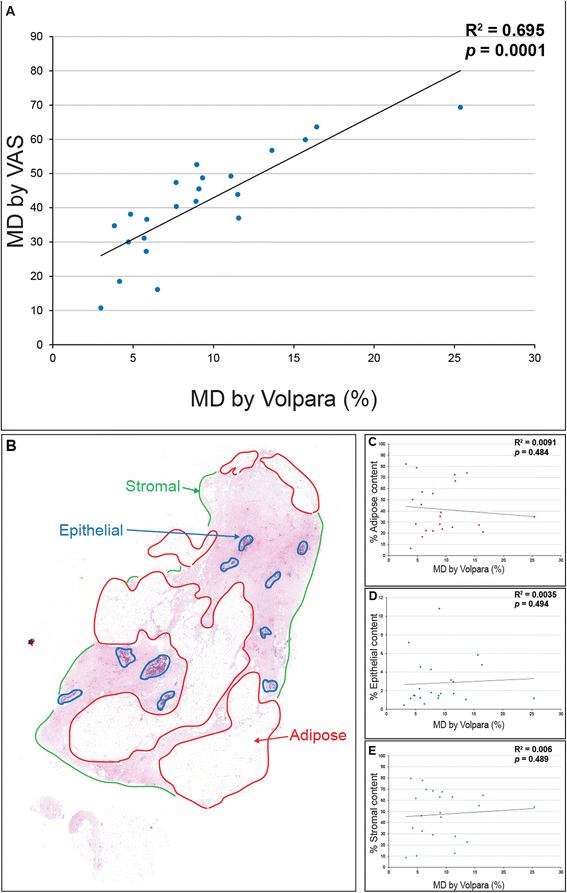
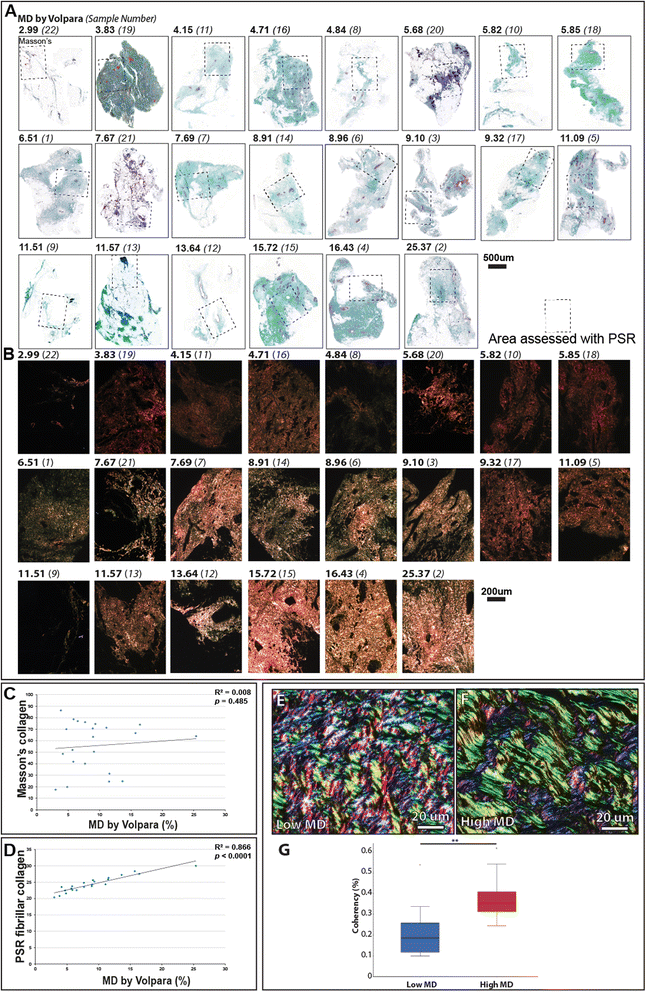
Results: Mammographic density was positively correlated with the abundance of aligned periductal fibrils rather than with the abundance of amorphous collagen. Compared with matched tissue resected from the breasts of low mammographic density patients, the highly birefringent tissue in mammographically dense breasts was both significantly stiffer and characterised by large (>80 μm long) fibrillar collagen bundles. Subsequent proteomic analyses not only confirmed the absence of collagen fibrosis in high mammographic density tissue, but additionally identified the up-regulation of periostin and collagen XVI (regulators of collagen fibril structure and architecture) as potential mediators of localised mechanical stiffness.
Conclusions: These preliminary data suggest that remodelling, and hence stiffening, of the existing stromal collagen microarchitecture promotes high mammographic density within the breast. In turn, this aberrant mechanical environment may trigger neoplasia-associated mechanotransduction pathways within the epithelial cell population.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

