Endothelial fibroblast growth factor receptor signaling is required for vascular remodeling following cardiac ischemia-reperfusion injury
- PMID: 26747503
- PMCID: PMC4796602
- DOI: 10.1152/ajpheart.00758.2015
Endothelial fibroblast growth factor receptor signaling is required for vascular remodeling following cardiac ischemia-reperfusion injury
Abstract
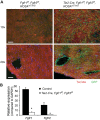
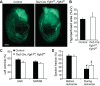
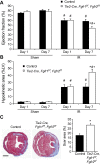
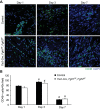
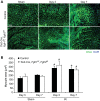
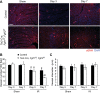
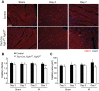
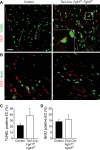
Fibroblast growth factor (FGF) signaling is cardioprotective in various models of myocardial infarction. FGF receptors (FGFRs) are expressed in multiple cell types in the adult heart, but the cell type-specific FGFR signaling that mediates different cardioprotective endpoints is not known. To determine the requirement for FGFR signaling in endothelium in cardiac ischemia-reperfusion injury, we conditionally inactivated the Fgfr1 and Fgfr2 genes in endothelial cells with Tie2-Cre (Tie2-Cre, Fgfr1(f/f), Fgfr2(f/f) DCKO mice). Tie2-Cre, Fgfr1(f/f), Fgfr2(f/f) DCKO mice had normal baseline cardiac morphometry, function, and vessel density. When subjected to closed-chest, regional cardiac ischemia-reperfusion injury, Tie2-Cre, Fgfr1(f/f), Fgfr2(f/f) DCKO mice showed a significantly increased hypokinetic area at 7 days, but not 1 day, after reperfusion. Tie2-Cre, Fgfr1(f/f), Fgfr2(f/f) DCKO mice also showed significantly worsened cardiac function compared with controls at 7 days but not 1 day after reperfusion. Pathophysiological analysis showed significantly decreased vessel density, increased endothelial cell apoptosis, and worsened tissue hypoxia in the peri-infarct area at 7 days following reperfusion. Notably, Tie2-Cre, Fgfr1(f/f), Fgfr2(f/f) DCKO mice showed no impairment in the cardiac hypertrophic response. These data demonstrate an essential role for FGFR1 and FGFR2 in endothelial cells for cardiac functional recovery and vascular remodeling following in vivo cardiac ischemia-reperfusion injury, without affecting the cardiac hypertrophic response. This study suggests the potential for therapeutic benefit from activation of endothelial FGFR pathways following ischemic injury to the heart.
Keywords: cardiac ischemia-reperfusion injury; endothelium; fibroblast growth factor; myocardial infarction; vascular remodeling.
Copyright © 2016 the American Physiological Society.
Figures

Similar articles
-
Fibroblast growth factor receptor signaling in cardiomyocytes is protective in the acute phase following ischemia-reperfusion injury.Front Cardiovasc Med. 2022 Sep 23;9:1011167. doi: 10.3389/fcvm.2022.1011167. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36211556 Free PMC article.
-
Endothelial cell FGF signaling is required for injury response but not for vascular homeostasis.Proc Natl Acad Sci U S A. 2014 Sep 16;111(37):13379-84. doi: 10.1073/pnas.1324235111. Epub 2014 Aug 19. Proc Natl Acad Sci U S A. 2014. PMID: 25139991 Free PMC article.
-
Endothelium-targeted overexpression of constitutively active FGF receptor induces cardioprotection in mice myocardial infarction.J Mol Cell Cardiol. 2009 May;46(5):663-73. doi: 10.1016/j.yjmcc.2009.01.015. J Mol Cell Cardiol. 2009. PMID: 19358330
-
Biological activities of fibroblast growth factor-2 in the adult myocardium.Cardiovasc Res. 2003 Jan;57(1):8-19. doi: 10.1016/s0008-6363(02)00708-3. Cardiovasc Res. 2003. PMID: 12504809 Review.
-
Fibroblast Growth Factor Receptors (FGFRs) and Noncanonical Partners in Cancer Signaling.Cells. 2021 May 14;10(5):1201. doi: 10.3390/cells10051201. Cells. 2021. PMID: 34068954 Free PMC article. Review.
Cited by
-
New developments in the biology of fibroblast growth factors.WIREs Mech Dis. 2022 Jul;14(4):e1549. doi: 10.1002/wsbm.1549. Epub 2022 Feb 9. WIREs Mech Dis. 2022. PMID: 35142107 Free PMC article. Review.
-
FGF2-induced STAT3 activation regulates pathologic neovascularization.Exp Eye Res. 2019 Oct;187:107775. doi: 10.1016/j.exer.2019.107775. Epub 2019 Aug 23. Exp Eye Res. 2019. PMID: 31449793 Free PMC article.
-
Fibroblast growth factors: the keepers of endothelial normalcy.J Clin Invest. 2021 Sep 1;131(17):e152716. doi: 10.1172/JCI152716. J Clin Invest. 2021. PMID: 34623324 Free PMC article.
-
Pathogenic variants damage cell composition and single cell transcription in cardiomyopathies.Science. 2022 Aug 5;377(6606):eabo1984. doi: 10.1126/science.abo1984. Epub 2022 Aug 5. Science. 2022. PMID: 35926050 Free PMC article.
-
Innovative hydrogel-based therapies for ischemia-reperfusion injury: bridging the gap between pathophysiology and treatment.Mater Today Bio. 2024 Oct 10;29:101295. doi: 10.1016/j.mtbio.2024.101295. eCollection 2024 Dec. Mater Today Bio. 2024. PMID: 39493810 Free PMC article. Review.
References
-
- Ahn A, Frishman WH, Gutwein A, Passeri J, Nelson M. Therapeutic angiogenesis a new treatment approach for ischemic heart disease– Part I. Cardiol Rev 16: 163–171, 2008. - PubMed
-
- Ahn A, Frishman WH, Gutwein A, Passeri J, Nelson M. Therapeutic angiogenesis a new treatment approach for ischemic heart disease–Part II. Cardiol Rev 16: 219–229, 2008. - PubMed
-
- Bastaki M, Nelli EE, Dell'Era P, Rusnati M, Molinari-Tosatti MP, Parolini S, Auerbach R, Ruco LP, Possati L, Presta M. Basic fibroblast growth factor-induced angiogenic phenotype in mouse endothelium. A study of aortic and microvascular endothelial cell lines. Arterioscler Thromb Vasc Biol 17: 454–464, 1997. - PubMed
-
- Bougioukas I, Didilis V, Ypsilantis P, Giatromanolaki A, Sivridis E, Lialiaris T, Mikroulis D, Simopoulos C, Bougioukas G. Intramyocardial injection of low-dose basic fibroblast growth factor or vascular endothelial growth factor induces angiogenesis in the infarcted rabbit myocardium. Cardiovasc Pathol 16: 63–68, 2007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

