Mincle suppresses Toll-like receptor 4 activation
- PMID: 26747838
- PMCID: PMC6608084
- DOI: 10.1189/jlb.3A0515-185R
Mincle suppresses Toll-like receptor 4 activation
Abstract
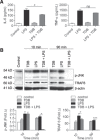
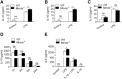
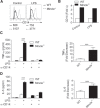
Regulation of Toll-like receptor responses is critical for limiting tissue injury and autoimmunity in both sepsis and sterile inflammation. We found that Mincle, a C-type lectin receptor, regulates proinflammatory Toll-like receptor 4 signaling. Specifically, Mincle ligation diminishes Toll-like receptor 4-mediated inflammation, whereas Mincle deletion or knockdown results in marked hyperresponsiveness to lipopolysaccharide in vitro, as well as overwhelming lipopolysaccharide-mediated inflammation in vivo. Mechanistically, Mincle deletion does not up-regulate Toll-like receptor 4 expression or reduce interleukin 10 production after Toll-like receptor 4 ligation; however, Mincle deletion decreases production of the p38 mitogen-activated protein kinase-dependent inhibitory intermediate suppressor of cytokine signaling 1, A20, and ABIN3 and increases expression of the Toll-like receptor 4 coreceptor CD14. Blockade of CD14 mitigates the increased sensitivity of Mincle(-/-) leukocytes to Toll-like receptor 4 ligation. Collectively, we describe a major role for Mincle in suppressing Toll-like receptor 4 responses and implicate its importance in nonmycobacterial models of inflammation.
Keywords: C-type lectin receptor; inflammation; sepsis.
© Society for Leukocyte Biology.
Figures





References
-
- Akira, S. , Takeda, K. (2004) Toll‐like receptor signalling. Nat. Rev. Immunol. 4, 499–511. - PubMed
-
- Kawai, T. , Akira, S. (2010) The role of pattern‐recognition receptors in innate immunity: update on Toll‐like receptors. Nat. Immunol. 11, 373–384. - PubMed
-
- Bianchi, M. E. (2007) DAMPs, PAMPs and alarmins: all we need to know about danger. J. Leukoc. Biol. 81, 1–5. - PubMed
-
- Kondo, T. , Kawai, T. , Akira, S. (2012) Dissecting negative regulation of Toll‐like receptor signaling. Trends Immunol. 33, 449–458. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

