Pharmacological targeting of CSF1R inhibits microglial proliferation and prevents the progression of Alzheimer's-like pathology
- PMID: 26747862
- PMCID: PMC4766375
- DOI: 10.1093/brain/awv379
Pharmacological targeting of CSF1R inhibits microglial proliferation and prevents the progression of Alzheimer's-like pathology
Abstract
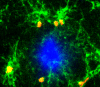
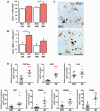
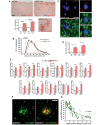
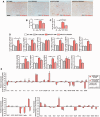
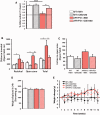
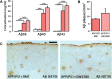
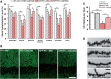
The proliferation and activation of microglial cells is a hallmark of several neurodegenerative conditions. This mechanism is regulated by the activation of the colony-stimulating factor 1 receptor (CSF1R), thus providing a target that may prevent the progression of conditions such as Alzheimer's disease. However, the study of microglial proliferation in Alzheimer's disease and validation of the efficacy of CSF1R-inhibiting strategies have not yet been reported. In this study we found increased proliferation of microglial cells in human Alzheimer's disease, in line with an increased upregulation of the CSF1R-dependent pro-mitogenic cascade, correlating with disease severity. Using a transgenic model of Alzheimer's-like pathology (APPswe, PSEN1dE9; APP/PS1 mice) we define a CSF1R-dependent progressive increase in microglial proliferation, in the proximity of amyloid-β plaques. Prolonged inhibition of CSF1R in APP/PS1 mice by an orally available tyrosine kinase inhibitor (GW2580) resulted in the blockade of microglial proliferation and the shifting of the microglial inflammatory profile to an anti-inflammatory phenotype. Pharmacological targeting of CSF1R in APP/PS1 mice resulted in an improved performance in memory and behavioural tasks and a prevention of synaptic degeneration, although these changes were not correlated with a change in the number of amyloid-β plaques. Our results provide the first proof of the efficacy of CSF1R inhibition in models of Alzheimer's disease, and validate the application of a therapeutic strategy aimed at modifying CSF1R activation as a promising approach to tackle microglial activation and the progression of Alzheimer's disease.
Keywords: Alzheimer’s disease; gliosis; inflammation; microglia; neurodegeneration.
© The Author (2016). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures

Comment in
-
Alzheimer disease: Blocking microglial proliferation halts Alzheimer disease in mice.Nat Rev Neurol. 2016 Feb;12(2):64. doi: 10.1038/nrneurol.2016.6. Epub 2016 Jan 22. Nat Rev Neurol. 2016. PMID: 26794653 No abstract available.
References
-
- Akiyama H, Nishimura T, Kondo H, Ikeda K, Hayashi Y, McGeer PL . Expression of the receptor for macrophage colony stimulating factor by brain microglia and its upregulation in brains of patients with Alzheimer's disease and amyotrophic lateral sclerosis . Brain Res 1994. ; 639 : 171 – 4 . - PubMed
-
- Boche D, Zotova E, Weller RO, Love S, Neal JW, Pickering RM, et al. . Consequence of Abeta immunization on the vasculature of human Alzheimer's disease brain . Brain 2008. ; 131 ( Pt 12 ): 3299 – 310 . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

