Post-Marketing Benefit-Risk Assessment of Rotavirus Vaccination in Japan: A Simulation and Modelling Analysis
- PMID: 26748506
- PMCID: PMC4749653
- DOI: 10.1007/s40264-015-0376-7
Post-Marketing Benefit-Risk Assessment of Rotavirus Vaccination in Japan: A Simulation and Modelling Analysis
Abstract
Introduction: Rotarix™, GSK's live attenuated rotavirus vaccine, was introduced in Japan in 2011. A recent trend in reduction of rotavirus gastroenteritis (RVGE) due to this vaccine was described. However, an observed/expected analysis showed a temporal association with intussusception within 7 days post dose 1.
Objective: In this paper, we compare the benefit and risk of vaccination side-by-side in a benefit-risk analysis.
Methods: The number of vaccine-preventable RVGE-associated hospitalizations and deaths (benefit) and intussusception-associated hospitalizations and deaths (risk) following two doses of Rotarix™ in Japan was compared using simulations. Source data included peer-reviewed clinical and epidemiological publications, Japanese governmental statistics (Statistics Bureau, Ministry of Internal Affairs and Communications), and market survey data.
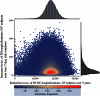
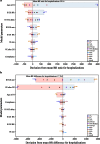
Results: For a birth cohort of 1 million vaccinated Japanese children followed for 5 years, the benefit-risk analysis suggested that the vaccine would prevent ~17,900 hospitalizations and ~6.3 deaths associated with RVGE. At the same time, vaccination would be associated with about ~50 intussusception hospitalizations and ~0.017 intussusception deaths. Therefore, for every intussusception hospitalization caused by vaccination and for one intussusception-associated death, 350 (95 % CI 69-2510) RVGE-associated hospitalizations and 366 (95 % CI 59-3271) RVGE-associated deaths are prevented, respectively, by vaccination.
Conclusions: The benefit-risk balance for Rotarix™ is favorable in Japan. From a public health perspective, the benefits in terms of prevented RVGE hospitalizations and deaths for the vaccinated population far exceed the estimated risks due to intussusception.
Figures

References
-
- Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12(2):136–141. doi: 10.1016/S1473-3099(11)70253-5. - DOI - PubMed
-
- Yokoo M, Arisawa K, Nakagomi O. Estimation of annual incidence, age-specific incidence rate, and cumulative risk of rotavirus gastroenteritis among children in Japan. Jpn J Infect Dis. 2004;57(4):166–171. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

