Encoding and decoding time in neural development
- PMID: 26748623
- PMCID: PMC11520978
- DOI: 10.1111/dgd.12257
Encoding and decoding time in neural development
Abstract
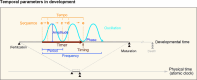
The development of a multicellular organism involves time-dependent changes in molecular and cellular states; therefore 'time' is an indispensable mathematical parameter of ontogenesis. Regardless of their inextricable relationship, there is a limited number of events for which the output of developmental phenomena primarily uses temporal cues that are generated through multilevel interactions between molecules, cells, and tissues. In this review, we focus on neural stem cells, which serve as a faithful decoder of temporal cues to transmit biological information and generate specific output in the developing nervous system. We further explore the identity of the temporal information that is encoded in neural development, and how this information is decoded into various cellular fate decisions.
Keywords: cell cycle; neural stem cell; temporal code; timing; transcriptional network.
© 2016 The Authors Development, Growth & Differentiation published by John Wiley & Sons Australia, Ltd on behalf of Japanese Society of Developmental Biologists.
Figures
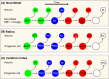


 CR cell,
CR cell,  Deep‐layer neuron,
Deep‐layer neuron,  Upper‐layer neuron.
Upper‐layer neuron.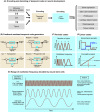
References
-
- Angevine, J. B. Jr & Sidman, R. L. 1961. Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature 192, 766–768. - PubMed
-
- Anthony, T. E. , Klein, C. , Fishell, G. & Heintz, N. 2004. Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron 41, 881–890. - PubMed
-
- Belliveau, M. J. & Cepko, C. L. 1999. Extrinsic and intrinsic factors control the genesis of amacrine and cone cells in the rat retina. Development 126, 555–566. - PubMed
-
- Berger, C. , Urban, J. & Technau, G. M. 2001. Stage‐specific inductive signals in the Drosophila neuroectoderm control the temporal sequence of neuroblast specification. Development 128, 3243–3251. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

