Proton detection and breathing regulation by the retrotrapezoid nucleus
- PMID: 26748771
- PMCID: PMC4799966
- DOI: 10.1113/JP271480
Proton detection and breathing regulation by the retrotrapezoid nucleus
Abstract
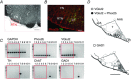
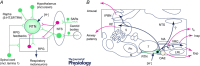
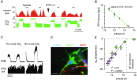
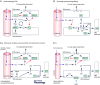
We discuss recent evidence which suggests that the principal central respiratory chemoreceptors are located within the retrotrapezoid nucleus (RTN) and that RTN neurons are directly sensitive to [H(+) ]. RTN neurons are glutamatergic. In vitro, their activation by [H(+) ] requires expression of a proton-activated G protein-coupled receptor (GPR4) and a proton-modulated potassium channel (TASK-2) whose transcripts are undetectable in astrocytes and the rest of the lower brainstem respiratory network. The pH response of RTN neurons is modulated by surrounding astrocytes but genetic deletion of RTN neurons or deletion of both GPR4 and TASK-2 virtually eliminates the central respiratory chemoreflex. Thus, although this reflex is regulated by innumerable brain pathways, it seems to operate predominantly by modulating the discharge rate of RTN neurons, and the activation of RTN neurons by hypercapnia may ultimately derive from their intrinsic pH sensitivity. RTN neurons increase lung ventilation by stimulating multiple aspects of breathing simultaneously. They stimulate breathing about equally during quiet wake and non-rapid eye movement (REM) sleep, and to a lesser degree during REM sleep. The activity of RTN neurons is regulated by inhibitory feedback and by excitatory inputs, notably from the carotid bodies. The latter input operates during normo- or hypercapnia but fails to activate RTN neurons under hypocapnic conditions. RTN inhibition probably limits the degree of hyperventilation produced by hypocapnic hypoxia. RTN neurons are also activated by inputs from serotonergic neurons and hypothalamic neurons. The absence of RTN neurons probably underlies the sleep apnoea and lack of chemoreflex that characterize congenital central hypoventilation syndrome.
© 2016 The Authors. The Journal of Physiology © 2016 The Physiological Society.
Figures


Similar articles
-
Central respiratory chemoreception.Handb Clin Neurol. 2022;188:37-72. doi: 10.1016/B978-0-323-91534-2.00007-2. Handb Clin Neurol. 2022. PMID: 35965033 Free PMC article. Review.
-
Intrinsic Molecular Proton Sensitivity Underlies GPR4 Effects on Retrotrapezoid Nucleus Neuronal Activation and CO2-Stimulated Breathing.J Neurosci. 2024 Sep 4;44(36):e0799242024. doi: 10.1523/JNEUROSCI.0799-24.2024. J Neurosci. 2024. PMID: 39107057 Free PMC article.
-
State-dependent control of breathing by the retrotrapezoid nucleus.J Physiol. 2015 Jul 1;593(13):2909-26. doi: 10.1113/JP270053. Epub 2015 May 22. J Physiol. 2015. PMID: 25820491 Free PMC article.
-
Hypoxia silences retrotrapezoid nucleus respiratory chemoreceptors via alkalosis.J Neurosci. 2015 Jan 14;35(2):527-43. doi: 10.1523/JNEUROSCI.2923-14.2015. J Neurosci. 2015. PMID: 25589748 Free PMC article.
-
The retrotrapezoid nucleus and breathing.Adv Exp Med Biol. 2012;758:115-22. doi: 10.1007/978-94-007-4584-1_16. Adv Exp Med Biol. 2012. PMID: 23080151 Free PMC article. Review.
Cited by
-
Phosphatidylinositol (4,5)-bisphosphate dynamically regulates the K2P background K+ channel TASK-2.Sci Rep. 2017 Mar 30;7:45407. doi: 10.1038/srep45407. Sci Rep. 2017. PMID: 28358046 Free PMC article.
-
Knockdown of PHOX2B in the retrotrapezoid nucleus reduces the central CO2 chemoreflex in rats.Elife. 2024 May 10;13:RP94653. doi: 10.7554/eLife.94653. Elife. 2024. PMID: 38727716 Free PMC article.
-
Brain metabolic sensing and metabolic signaling at the level of an astrocyte.Glia. 2018 Jun;66(6):1185-1199. doi: 10.1002/glia.23283. Epub 2017 Dec 23. Glia. 2018. PMID: 29274121 Free PMC article. Review.
-
Criteria for central respiratory chemoreceptors: experimental evidence supporting current candidate cell groups.Front Physiol. 2023 Sep 1;14:1241662. doi: 10.3389/fphys.2023.1241662. eCollection 2023. Front Physiol. 2023. PMID: 37719465 Free PMC article. Review.
-
Central respiratory chemoreception.Handb Clin Neurol. 2022;188:37-72. doi: 10.1016/B978-0-323-91534-2.00007-2. Handb Clin Neurol. 2022. PMID: 35965033 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

