Tuning microenvironment modulus and biochemical composition promotes human mesenchymal stem cell tenogenic differentiation
- PMID: 26748903
- PMCID: PMC5510610
- DOI: 10.1002/jbm.a.35650
Tuning microenvironment modulus and biochemical composition promotes human mesenchymal stem cell tenogenic differentiation
Abstract
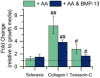
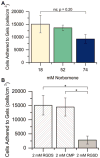
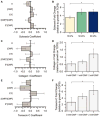
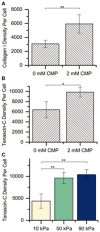
Mesenchymal stem cells (MSCs) are promising for the regeneration of tendon and ligament tissues. Toward realizing this potential, microenvironment conditions are needed for promoting robust lineage-specific differentiation into tenocytes/ligament fibroblasts. Here, we utilized a statistical design of experiments approach to examine combinations of matrix modulus, composition, and soluble factors in human MSC tenogenic/ligamentogenic differentiation. Specifically, well-defined poly(ethylene glycol)-based hydrogels were synthesized using thiol-ene chemistry providing a bioinert base for probing cell response to extracellular matrix cues. Monomer concentrations were varied to achieve a range of matrix moduli (E ∼ 10-90 kPa), and different ratios of integrin-binding peptides were incorporated (GFOGER and RGDS for collagen and fibronectin, respectively), mimicking aspects of developing tendon/ligament tissue. A face-centered central composite response surface design was utilized to understand the contributions of these cues to human MSC differentiation in the presence of soluble factors identified to promote tenogenesis/ligamentogenesis (BMP-13 and ascorbic acid). Increasing modulus and collagen mimetic peptide content increased relevant gene expression and protein production or retention (scleraxis, collagen I, tenascin-C). These findings could inform the design of materials for tendon/ligament regeneration. More broadly, the design of experiments enabled efficient data acquisition and analysis, requiring fewer replicates than if each factor had been varied one at a time. This approach can be combined with other stimuli (for example, mechanical stimulation) toward a better mechanistic understanding of differentiation down these challenging lineages.
Keywords: cell microenvironment; design of experiments; hydrogel; mesenchymal stem cell; tendon.
© 2016 Wiley Periodicals, Inc.
Figures


Similar articles
-
Interplay between degradability and integrin signaling on mesenchymal stem cell function within poly(ethylene glycol) based microporous annealed particle hydrogels.Acta Biomater. 2020 Jan 1;101:227-236. doi: 10.1016/j.actbio.2019.11.009. Epub 2019 Nov 8. Acta Biomater. 2020. PMID: 31711899 Free PMC article.
-
Growth Factor-Mediated Tenogenic Induction of Multipotent Mesenchymal Stromal Cells Is Altered by the Microenvironment of Tendon Matrix.Cell Transplant. 2018 Oct;27(10):1434-1450. doi: 10.1177/0963689718792203. Epub 2018 Sep 25. Cell Transplant. 2018. PMID: 30251565 Free PMC article.
-
Tendon Tissue Engineering: Effects of Mechanical and Biochemical Stimulation on Stem Cell Alignment on Cell-Laden Hydrogel Yarns.Adv Healthc Mater. 2019 Apr;8(7):e1801218. doi: 10.1002/adhm.201801218. Epub 2019 Feb 6. Adv Healthc Mater. 2019. PMID: 30725521
-
Tendon tissue engineering: Current progress towards an optimized tenogenic differentiation protocol for human stem cells.Acta Biomater. 2022 Jun;145:25-42. doi: 10.1016/j.actbio.2022.04.028. Epub 2022 Apr 22. Acta Biomater. 2022. PMID: 35470075 Review.
-
Mesenchymal Stem Cells Empowering Tendon Regenerative Therapies.Int J Mol Sci. 2019 Jun 19;20(12):3002. doi: 10.3390/ijms20123002. Int J Mol Sci. 2019. PMID: 31248196 Free PMC article. Review.
Cited by
-
Hierarchy Reproduction: Multiphasic Strategies for Tendon/Ligament-Bone Junction Repair.Biomater Res. 2025 Jan 22;29:0132. doi: 10.34133/bmr.0132. eCollection 2025. Biomater Res. 2025. PMID: 39844867 Free PMC article. Review.
-
Predicting Ligand-Free Cell Attachment on Next-Generation Cellulose-Chitosan Hydrogels.ACS Omega. 2018 Jan 31;3(1):937-945. doi: 10.1021/acsomega.7b01583. Epub 2018 Jan 25. ACS Omega. 2018. PMID: 30023793 Free PMC article.
-
Rate-based approach for controlling the mechanical properties of 'thiol-ene' hydrogels formed with visible light.Polym Chem. 2019 Aug 28;10(32):4428-4440. doi: 10.1039/C9PY00447E. Epub 2019 Jul 8. Polym Chem. 2019. PMID: 32405326 Free PMC article.
-
Systematic d-Amino Acid Substitutions to Control Peptide and Hydrogel Degradation in Cellular Microenvironments.ACS Macro Lett. 2023 Jun 20;12(6):725-732. doi: 10.1021/acsmacrolett.3c00144. Epub 2023 May 17. ACS Macro Lett. 2023. PMID: 37195203 Free PMC article.
-
Designing Microgels for Cell Culture and Controlled Assembly of Tissue Microenvironments.Adv Funct Mater. 2020 Sep 10;30(37):1907670. doi: 10.1002/adfm.201907670. Epub 2019 Dec 17. Adv Funct Mater. 2020. PMID: 33841061 Free PMC article.
References
-
- Kartus J, Movin T, Karlsson J. Donor-site morbidity and anterior knee problems after anterior cruciate ligament reconstruction using autografts. Arthroscopy. 2001;17:971–80. - PubMed
-
- Rodrigues MT, Reis RL, Gomes ME. Engineering tendon and ligament tissues: present developments towards successful clinical products. J Tissue Eng Regen Med. 2013;7:673–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

