Presentation of high antigen-dose by splenic B220(lo) B cells fosters a feedback loop between T helper type 2 memory and antibody isotype switching
- PMID: 26749165
- PMCID: PMC4799881
- DOI: 10.1111/imm.12579
Presentation of high antigen-dose by splenic B220(lo) B cells fosters a feedback loop between T helper type 2 memory and antibody isotype switching
Abstract
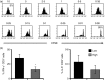
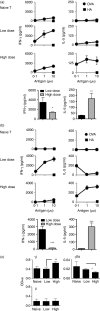
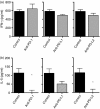
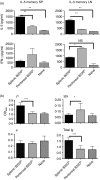
Effective humoral immunity ensues when antigen presentation by B cells culminates in productive cooperation with T lymphocytes. This collaboration, however, remains ill-defined because naive antigen-specific B cells are rare and difficult to track in vivo. Herein, we used a defined transfer model to examine how B lymphocytes, as antigen-presenting cells, shape the development of T-cell memory suitable for generation of relevant antibody responses. Specifically, we examined how B cells presenting different doses of antigen during the initial priming phase shape the development of CD4 T-cell memory and its influence on humoral immunity. The findings indicate that B cells presenting low dose of antigen favour the development of T helper type 1 (Th1) type memory, while those presenting a high antigen dose yielded better Th2 memory cells. The memory Th2 cells supported the production of antibodies by effector B cells and promoted isotype switching to IgG1. Moreover, among the B-cell subsets tested for induction of Th2 memory, the splenic but not peritoneal B220(lo) cells were most effective in sustaining Th2 memory development as well as immunoglobulin isotype switching, and this function involved a tight control by programmed death 1-programmed death ligand 2 interactions.
Keywords: B cells; T/B-cell collaboration; antigen presentation; co-stimulatory molecules.
© 2016 John Wiley & Sons Ltd.
Figures




References
-
- Chen X, Jensen PE. The role of B lymphocytes as antigen‐presenting cells. Arch Immunol Ther Exp (Warsz) 2008; 56:77–83. - PubMed
-
- Delves PJ, Roitt IM. The immune system. First of two parts. N Engl J Med 2000; 343:37–49. - PubMed
-
- Evans DE, Munks MW, Purkerson JM, Parker DC. Resting B lymphocytes as APC for naive T lymphocytes: dependence on CD40 ligand/CD40. J Immunol 2000; 164:688–97. - PubMed
-
- Lassila O, Vainio O, Matzinger P. Can B cells turn on virgin T cells? Nature 1988; 334:253–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

