Expanding the Landscape of Diterpene Structural Diversity through Stereochemically Controlled Combinatorial Biosynthesis
- PMID: 26749264
- PMCID: PMC4755150
- DOI: 10.1002/anie.201510650
Expanding the Landscape of Diterpene Structural Diversity through Stereochemically Controlled Combinatorial Biosynthesis
Abstract
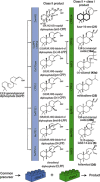
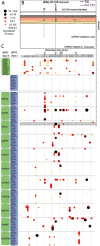
Plant-derived diterpenoids serve as important pharmaceuticals, food additives, and fragrances, yet their low natural abundance and high structural complexity limits their broader industrial utilization. By mimicking the modularity of diterpene biosynthesis in plants, we constructed 51 functional combinations of class I and II diterpene synthases, 41 of which are "new-to-nature". Stereoselective biosynthesis of over 50 diterpene skeletons was demonstrated, including natural variants and novel enantiomeric or diastereomeric counterparts. Scalable biotechnological production for four industrially relevant targets was accomplished in engineered strains of Saccharomyces cerevisiae.
Keywords: biocatalysis; biosynthesis; combinatorial biosynthesis; cyclizations; terpenes.
© 2015 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Figures

References
-
- None
-
- Paddon C. J., et al., Nature 2013, 496, 528; - PubMed
-
- Holton R. A., Kim H. B., Somoza C., Liang F., Biediger R. J., Boatman P. D., Shindo M., Smith C. C., Kim S., J. Am. Chem. Soc. 1994, 116, 1599;
-
- Jørgensen L., McKerrall S. J., Kuttruff C. A., Ungeheuer F., Felding J., Baran P. S., Science 2013, 341, 878. - PubMed

