How Accurate is Your Sclerostin Measurement? Comparison Between Three Commercially Available Sclerostin ELISA Kits
- PMID: 26749312
- PMCID: PMC4860200
- DOI: 10.1007/s00223-015-0105-3
How Accurate is Your Sclerostin Measurement? Comparison Between Three Commercially Available Sclerostin ELISA Kits
Abstract
Sclerostin, bone formation antagonist is in the spotlight as a potential biomarker for diseases presenting with associated bone disorders such as chronic kidney disease (CDK-MBD). Accurate measurement of sclerostin is therefore important. Several immunoassays are available to measure sclerostin in serum and plasma. We compared the performance of three commercial ELISA kits. We measured sclerostin concentrations in serum and EDTA plasma obtained from healthy young (18-26 years) human subjects using kits from Biomedica, TECOmedical and from R&D Systems. The circulating sclerostin concentrations were systematically higher when measured with the Biomedica assay (serum: 35.5 ± 1.1 pmol/L; EDTA: 39.4 ± 2.0 pmol/L; mean ± SD) as compared with TECOmedical (serum: 21.8 ± 0.7 pmol/L; EDTA: 27.2 ± 1.3 pmol/L) and R&D Systems (serum: 7.6 ± 0.3 pmol/L; EDTA: 30.9 ± 1.5 pmol/L). We found a good correlation between the assay for EDTA plasma (r > 0.6; p < 0.001) while in serum, only measurements obtained using TECOmedical and R&D Systems assays correlated significantly (r = 0.78; p < 0.001). There was no correlation between matrices results when using the Biomedica kit (r = 0.20). The variability in values generated from Biomedica, R&D Systems and TECOmedical assays raises questions regarding the accuracy and specificity of the assays. Direct comparison of studies using different kits is not possible and great care should be given to measurement of sclerostin, with traceability of reagents. Standardization with appropriate material is required before different sclerostin assays can be introduced in clinical practice.
Keywords: Clinical utility; ELISA; Metabolic bone disease; Sclerostin.
Figures

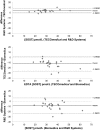
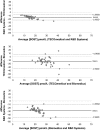
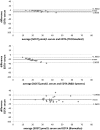
Comment in
-
Serum Sclerostin: Not Only a Matter of Measurement But Also of Meaning.Calcif Tissue Int. 2016 Jun;98(6):642-3. doi: 10.1007/s00223-016-0115-9. Epub 2016 Feb 12. Calcif Tissue Int. 2016. PMID: 26873477 No abstract available.
Similar articles
-
Comparison of two commercially available ELISAs for circulating sclerostin.Osteoporos Int. 2014 May;25(5):1547-54. doi: 10.1007/s00198-014-2635-3. Epub 2014 Feb 22. Osteoporos Int. 2014. PMID: 24562839
-
Determination of serum and plasma sclerostin concentrations by enzyme-linked immunoassays.J Clin Endocrinol Metab. 2011 Jul;96(7):E1159-62. doi: 10.1210/jc.2011-0254. Epub 2011 May 4. J Clin Endocrinol Metab. 2011. PMID: 21543425 Free PMC article.
-
Factors associated with serum soluble inhibitors of Wnt-β-catenin signaling (sclerostin and dickkopf-1) in patients undergoing peritoneal dialysis.Nephrology (Carlton). 2015 Sep;20(9):639-45. doi: 10.1111/nep.12509. Nephrology (Carlton). 2015. PMID: 25974190
-
Sclerostin measurement in human disease: Validity and current limitations.Bone. 2017 Mar;96:24-28. doi: 10.1016/j.bone.2016.10.012. Epub 2016 Oct 11. Bone. 2017. PMID: 27742501 Review.
-
[Serum sclerostin levels and metabolic bone diseases].Clin Calcium. 2013 Jun;23(6):877-83. Clin Calcium. 2013. PMID: 23719501 Review. Japanese.
Cited by
-
Circulating sclerostin is associated with bone mineral density independent of HIV-serostatus.Bone Rep. 2020 May 11;12:100279. doi: 10.1016/j.bonr.2020.100279. eCollection 2020 Jun. Bone Rep. 2020. PMID: 32455152 Free PMC article.
-
Sclerostin: a new biomarker of CKD-MBD.Int Urol Nephrol. 2020 Jan;52(1):107-113. doi: 10.1007/s11255-019-02290-3. Epub 2019 Oct 14. Int Urol Nephrol. 2020. PMID: 31612420 Review.
-
SOST silencing promotes proliferation and invasion and reduces apoptosis of retinoblastoma cells by activating Wnt/β-catenin signaling pathway.Gene Ther. 2017 Jul;24(7):399-407. doi: 10.1038/gt.2017.31. Epub 2017 Jun 15. Gene Ther. 2017. PMID: 28485721
-
Validation of a novel, rapid, high precision sclerostin assay not confounded by sclerostin fragments.Bone. 2018 Jun;111:36-43. doi: 10.1016/j.bone.2018.03.013. Epub 2018 Mar 14. Bone. 2018. PMID: 29550267 Free PMC article.
-
Gender-Affirming Hormone Treatment Decreases Bone Turnover in Transwomen and Older Transmen.J Bone Miner Res. 2019 Oct;34(10):1862-1872. doi: 10.1002/jbmr.3762. Epub 2019 Aug 19. J Bone Miner Res. 2019. PMID: 31099910 Free PMC article. Clinical Trial.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

