Anticancer efficacy of the hypoxia-activated prodrug evofosfamide (TH-302) in osteolytic breast cancer murine models
- PMID: 26749324
- PMCID: PMC4799961
- DOI: 10.1002/cam4.599
Anticancer efficacy of the hypoxia-activated prodrug evofosfamide (TH-302) in osteolytic breast cancer murine models
Abstract
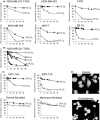
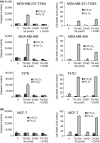
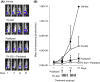
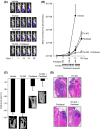
Tumor hypoxia is a major cause of treatment failure for a variety of malignancies. However, hypoxia offers treatment opportunities, exemplified by the development of compounds that target hypoxic regions within tumors. Evofosfamide (TH-302) is a prodrug created by the conjugation of 2-nitroimidazole to bromo-isophosphoramide mustard (Br-IPM). When evofosfamide is delivered to hypoxic regions, the DNA cross-linking effector, Br-IPM, is released. This study assessed the cytotoxic activity of evofosfamide in vitro and its antitumor activity against osteolytic breast cancer either alone or in combination with paclitaxel in vivo. A panel of human breast cancer cell lines were treated with evofosfamide under hypoxia and assessed for cell viability. Osteolytic MDA-MB-231-TXSA cells were transplanted into the mammary fat pad, or into tibiae of mice, allowed to establish and treated with evofosfamide, paclitaxel, or both. Tumor burden was monitored using bioluminescence, and cancer-induced bone destruction was measured using micro-CT. In vitro, evofosfamide was selectively cytotoxic under hypoxic conditions. In vivo evofosfamide was tumor suppressive as a single agent and cooperated with paclitaxel to reduce mammary tumor growth. Breast cancer cells transplanted into the tibiae of mice developed osteolytic lesions. In contrast, treatment with evofosfamide or paclitaxel resulted in a significant delay in tumor growth and an overall reduction in tumor burden in bone, whereas combined treatment resulted in a significantly greater reduction in tumor burden in the tibia of mice. Evofosfamide cooperates with paclitaxel and exhibits potent tumor suppressive activity against breast cancer growth in the mammary gland and in bone.
Keywords: Breast cancer; TH-302; chemotherapy; evofosfamide; hypoxia; tumor growth.
© 2016 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Figures

Similar articles
-
Anticancer efficacy of the hypoxia-activated prodrug evofosfamide is enhanced in combination with proapoptotic receptor agonists against osteosarcoma.Cancer Med. 2017 Sep;6(9):2164-2176. doi: 10.1002/cam4.1115. Epub 2017 Aug 10. Cancer Med. 2017. PMID: 28799237 Free PMC article.
-
Hypoxia-activated pro-drug TH-302 exhibits potent tumor suppressive activity and cooperates with chemotherapy against osteosarcoma.Cancer Lett. 2015 Feb 1;357(1):160-169. doi: 10.1016/j.canlet.2014.11.020. Epub 2014 Nov 15. Cancer Lett. 2015. PMID: 25444931 Free PMC article.
-
Efficacy of the hypoxia-activated prodrug evofosfamide (TH-302) in nasopharyngeal carcinoma in vitro and in vivo.Cancer Commun (Lond). 2018 May 3;38(1):15. doi: 10.1186/s40880-018-0285-0. Cancer Commun (Lond). 2018. PMID: 29764490 Free PMC article.
-
Evofosfamide, a new horizon in the treatment of pancreatic cancer.Anticancer Drugs. 2016 Sep;27(8):723-5. doi: 10.1097/CAD.0000000000000386. Anticancer Drugs. 2016. PMID: 27232101 Review.
-
Bioreductive prodrugs as cancer therapeutics: targeting tumor hypoxia.Chin J Cancer. 2014 Feb;33(2):80-6. doi: 10.5732/cjc.012.10285. Epub 2013 Jul 12. Chin J Cancer. 2014. PMID: 23845143 Free PMC article. Review.
Cited by
-
An Orally Available Tubulin Inhibitor, VERU-111, Suppresses Triple-Negative Breast Cancer Tumor Growth and Metastasis and Bypasses Taxane Resistance.Mol Cancer Ther. 2020 Feb;19(2):348-363. doi: 10.1158/1535-7163.MCT-19-0536. Epub 2019 Oct 23. Mol Cancer Ther. 2020. PMID: 31645441 Free PMC article.
-
Engineered extracellular vesicles for combinatorial TNBC therapy: SR-SIM-guided design achieves substantial drug dosage reduction.Mol Ther. 2024 Dec 4;32(12):4467-4481. doi: 10.1016/j.ymthe.2024.09.034. Epub 2024 Oct 5. Mol Ther. 2024. PMID: 39369270
-
Evofosfamide sensitizes esophageal carcinomas to radiation without increasing normal tissue toxicity.Radiother Oncol. 2019 Dec;141:247-255. doi: 10.1016/j.radonc.2019.06.034. Epub 2019 Aug 17. Radiother Oncol. 2019. PMID: 31431383 Free PMC article.
-
Development of a cobalt(iii)-based ponatinib prodrug system.Inorg Chem Front. 2021 Mar 30;8(10):2468-2485. doi: 10.1039/d1qi00211b. Inorg Chem Front. 2021. PMID: 34046181 Free PMC article.
-
Phase IB study of sorafenib and evofosfamide in patients with advanced hepatocellular and renal cell carcinomas (NCCTG N1135, Alliance).Invest New Drugs. 2021 Aug;39(4):1072-1080. doi: 10.1007/s10637-021-01090-w. Epub 2021 Mar 1. Invest New Drugs. 2021. PMID: 33646489 Free PMC article. Clinical Trial.
References
-
- Metin Seker, M. , Yucel B., Seker A., Ay Eren A., Bahar S., Celasun G., et al. 2014. Treatment and prognosis of breast cancer in elderly: different from young patients? Eur. Ger. Med. 5:261–264.
-
- Mundy, G. R . 2002. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat. Rev. Cancer 2: 584–593. - PubMed
-
- Brown, J. M . 2000. Exploiting the hypoxic cancer cell: mechanisms and therapeutic strategies. Mol. Med. Today 6:157–162. - PubMed
-
- Melillo, G . 2007. Targeting hypoxia cell signaling for cancer therapy. Cancer Metastasis Rev. 26: 341–352. - PubMed
-
- Duan, J.‐J. , Jiao H., Kaizerman J., Stanton T., Evans J. W., Lan L., et al. 2007. Potent and highly selective hypoxia‐activated achiral phosphoramidate mustards as anticancer drugs. J. Med. Chem. 51: 2412–2420. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

