Developments in Varicella Zoster Virus Vasculopathy
- PMID: 26750127
- PMCID: PMC5489063
- DOI: 10.1007/s11910-015-0614-5
Developments in Varicella Zoster Virus Vasculopathy
Abstract
Varicella zoster virus (VZV) is a highly neurotropic human herpesvirus. Primary infection usually causes varicella (chicken pox), after which virus becomes latent in ganglionic neurons along the entire neuraxis. VZV reactivation results in zoster (shingles) which is frequently complicated by chronic pain (postherpetic neuralgia). VZV reactivation also causes meningoencephalitis, myelitis, ocular disorders, and vasculopathy, all of which can occur in the absence of rash. This review focuses on the association of VZV and stroke, and on the widening spectrum of disorders produced by VZV vasculopathy in immunocompetent and immunocompromised individuals, including recipients of varicella vaccine. Aside from ischemic stroke, VZV infection of cerebral arteries may lead to development of intracerebral aneurysms, with or without hemorrhage. Moreover, recent clinical-virological case reports and retrospective pathological-virological analyses of temporal arteries positive or negative for giant cell arteritis (GCA) indicate that extracranial VZV vasculopathy triggers the immunopathology of GCA. While many patients with GCA improve after corticosteroid treatment, prolonged corticosteroid use may potentiate VZV infection, leading to fatal vasculopathy in the brain and other organs.
Keywords: Aneurysm; Giant cell arteritis; Stroke; Varicella zoster virus vasculopathy; Zoster.
Conflict of interest statement
Figures
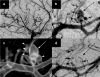

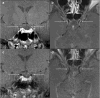
References
-
- Baudouin E, Lantuejoul P. Les troublecas moteurs dans le zona. Gazette des Hopitaux. 1919 The first description of stroke associated with VZV.
-
- Nagel MA, Forghani B, Mahalingam R, et al. The value of detecting anti-VZV IgG antibody in CSF to diagnose VZV vasculopathy. Neurology. 2007;68:1069–73. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

