Asymmetric synthesis of cyclopentanes bearing four contiguous stereocenters via an NHC-catalyzed Michael/Michael/esterification domino reaction
- PMID: 26750327
- PMCID: PMC5315016
- DOI: 10.1039/c5cc09581f
Asymmetric synthesis of cyclopentanes bearing four contiguous stereocenters via an NHC-catalyzed Michael/Michael/esterification domino reaction
Abstract
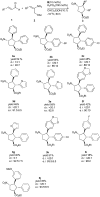
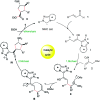
An NHC-catalyzed Michael/Michael/esterification domino reaction via homoenolate/enolate intermediates for the asymmetric synthesis of tetrasubstituted cyclopentanes bearing four contiguous stereocenters is described. A variety of α,β-unsaturated aldehydes and 2-nitroallylic acetates react well with good domino yields and high stereoselectivities.
Figures


Similar articles
-
Asymmetric Synthesis of Functionalized Tricyclic Chromanes via an Organocatalytic Triple Domino Reaction.Org Lett. 2017 Jun 2;19(11):3025-3028. doi: 10.1021/acs.orglett.7b01322. Epub 2017 May 23. Org Lett. 2017. PMID: 28534639
-
Asymmetric synthesis of functionalized tetrahydrofluorenones via an NHC-catalyzed homoenolate Michael addition.Chem Commun (Camb). 2018 Jul 14;54(55):7661-7664. doi: 10.1039/c8cc04145h. Epub 2018 Jun 22. Chem Commun (Camb). 2018. PMID: 29932187
-
Organocatalytic Asymmetric Synthesis of Functionalized 1,3,5-Triarylpyrrolidin-2-ones via an Aza-Michael/Aldol Domino Reaction.Synthesis (Stuttg). 2014 Mar 1;46(6):799-808. doi: 10.1055/s-0033-1340565. Synthesis (Stuttg). 2014. PMID: 25278634 Free PMC article.
-
NHC-Catalyzed Generation of α,β-Unsaturated Acylazoliums for the Enantioselective Synthesis of Heterocycles and Carbocycles.Acc Chem Res. 2019 Feb 19;52(2):425-436. doi: 10.1021/acs.accounts.8b00550. Epub 2019 Jan 17. Acc Chem Res. 2019. PMID: 30653296
-
An organocatalytic domino Michael addition strategy: construction of bispiro[oxindole-thiazolidinone-hexahydroxanthone]s with five contiguous stereocenters.Org Biomol Chem. 2020 Sep 30;18(37):7373-7378. doi: 10.1039/d0ob01613f. Org Biomol Chem. 2020. PMID: 32926035
Cited by
-
Organocatalytic Name Reactions Enabled by NHCs.Materials (Basel). 2020 Aug 13;13(16):3574. doi: 10.3390/ma13163574. Materials (Basel). 2020. PMID: 32823580 Free PMC article. Review.
-
N-Heterocyclic-Carbene-Catalyzed Domino Reactions via Two or More Activation Modes.iScience. 2018 Apr 27;2:1-26. doi: 10.1016/j.isci.2018.03.006. Epub 2018 Apr 5. iScience. 2018. PMID: 30428366 Free PMC article. Review.
-
Multicomponent reaction (MCR) for constructing bis-spirocyclohexane skeletons using β-nitrostyrene derived MBH acetates, 1,3-indanedione and aldehydes via [1 + 1 + 1 + 3] annulation.RSC Adv. 2023 Sep 13;13(39):27456-27460. doi: 10.1039/d3ra04996e. eCollection 2023 Sep 8. RSC Adv. 2023. PMID: 37711377 Free PMC article.
-
Synthesis of tetrahydrochromenes and dihydronaphthofurans via a cascade process of [3 + 3] and [3 + 2] annulation reactions: mechanistic insight for 6-endo-trig and 5-exo-trig cyclisation.RSC Adv. 2023 Feb 16;13(9):5796-5803. doi: 10.1039/d2ra08163f. eCollection 2023 Feb 14. RSC Adv. 2023. PMID: 36816068 Free PMC article.
References
-
- Bestmann H. J., Roth D. Synlett. 1990:751.
- Boyer S. J., Leahy J. W. J. Org. Chem. 1997;62:3976.
-
- Whitson J. T. Expert Opin. Pharmacother. 2002;3:965. - PubMed
-
- Babu Y. S., Chand P., Bantia S., Kotian P., Dehghani A., El-Kattan Y., Lin T.-H., Hutchison T. L., Elliott A. J., Parker C. D., Ananth S. L., Horn L. L., Laver G. W., Montgomery J. A. J. Med. Chem. 2000;43:3482. - PubMed
- Chand P., Kotian P. L., Dehghani A., El-Kattan Y., Lin T.-H., Hutchison T. L., Babu Y. S., Bantia S., Elliott A. J., Montgomery J. A. J. Med. Chem. 2001;44:4379. - PubMed
- Chand P., Babu Y. S., Bantia S., Rowland S., Dehghani A., Kotian P. L., Hutchison T. L., Ali S., Brouillette W., El-Kattan Y., Lin T.-H. J. Med. Chem. 2004;47:1919. - PubMed
-
- Hudlicky T., Price J. D. Chem. Rev. 1989;89:1467.
- Lautens M., Klute W., Tam W. Chem. Rev. 1996;96:49. - PubMed
- Heasley B. Eur. J. Org. Chem. 2009:1477.
- Nair V., Babu B. P., Vellalath S., Varghese V., Raveendran A. E., Suresh E. Org. Lett. 2009;11:2507. - PubMed
- Chiang P.-C., Rommel M., Bode J. W. J. Am. Chem. Soc. 2009;131:8714. - PMC - PubMed
- Kaeobamrung J., Bode J. W. Org. Lett. 2009;11:677. - PMC - PubMed
- Cohen D. T., Cardinal-David B., Scheidt K. A. Angew. Chem., Int. Ed. 2011;50:1678. - PMC - PubMed
- Liu G., Shirley M. E., Van K. N., McFarlin R. L., Romo D. Nat. Chem. 2013;5:1049. - PMC - PubMed
- Parr B. T., Davies H. M. L. Nat. Commun. 2014;5:1. - PMC - PubMed
- Zou L.-H., Philipps A. R., Raabe G., Enders D. Chem. – Eur. J. 2015;21:1004. - PMC - PubMed
- Mukherjee S., Mondal S., Patra A., Gonnadeb R. G., Biju A. T. Chem. Commun. 2015;51:9559. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

