Downregulation of Organic Anion Transporting Polypeptide (OATP) 1B1 Transport Function by Lysosomotropic Drug Chloroquine: Implication in OATP-Mediated Drug-Drug Interactions
- PMID: 26750564
- PMCID: PMC4970216
- DOI: 10.1021/acs.molpharmaceut.5b00763
Downregulation of Organic Anion Transporting Polypeptide (OATP) 1B1 Transport Function by Lysosomotropic Drug Chloroquine: Implication in OATP-Mediated Drug-Drug Interactions
Abstract
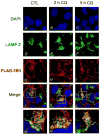
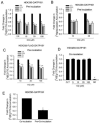
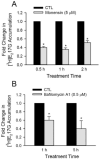
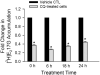
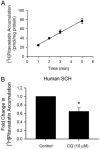
Organic anion transporting polypeptide (OATP) 1B1 mediates the hepatic uptake of many drugs including lipid-lowering statins. Decreased OATP1B1 transport activity is often associated with increased systemic exposure of statins and statin-induced myopathy. Antimalarial drug chloroquine (CQ) is also used for long-term treatment of rheumatoid arthritis and systemic lupus erythematosus. CQ is lysosomotropic and inhibits protein degradation in lysosomes. The current studies were designed to determine the effects of CQ on OATP1B1 protein degradation, OATP1B1-mediated transport in OATP1B1-overexpressing cell line, and statin uptake in human sandwich-cultured hepatocytes (SCH). Treatment with lysosome inhibitor CQ increased OATP1B1 total protein levels in HEK293-OATP1B1 cells and in human SCH as determined by OATP1B1 immunoblot. In HEK293-FLAG-tagged OATP1B1 stable cell line, co-immunofluorescence staining indicated that intracellular FLAG-OATP1B1 is colocalized with lysosomal associated membrane glycoprotein (LAMP)-2, a marker protein of late endosome/lysosome. Enlarged LAMP-2-positive vacuoles with FLAG-OATP1B1 protein retained inside were readily detected in CQ-treated cells, consistent with blocking lysosomal degradation of OATP1B1 by CQ. In HEK293-OATP1B1 cells, without pre-incubation, CQ concentrations up to 100 μM did not affect OATP1B1-mediated [(3)H]E217G accumulation. However, pre-incubation with CQ at clinically relevant concentration(s) significantly decreased [(3)H]E217G and [(3)H]pitavastatin accumulation in HEK293-OATP1B1 cells and [(3)H]pitavastatin accumulation in human SCH. CQ pretreatment (25 μM, 2 h) resulted in ∼1.9-fold decrease in Vmax without affecting Km of OATP1B1-mediated [(3)H]E217G transport in HEK293-OATP1B1 cells. Pretreatment with monensin and bafilomycin A1, which also have lysosome inhibition activity, significantly decreased OATP1B1-mediated transport in HEK293-OATP1B1 cells. Pharmacoepidemiologic studies using data from the U.S. Food and Drug Administration Adverse Event Reporting System indicated that CQ plus pitavastatin, rosuvastatin, and pravastatin, which are minimally metabolized by the cytochrome P450 enzymes, led to higher myopathy risk than these statins alone. In summary, the present studies report novel findings that lysosome is involved in degradation of OATP1B1 protein and that pre-incubation with lysosomotropic drug CQ downregulates OATP1B1 transport activity. Our in vitro data in combination with pharmacoepidemiologic studies support that CQ has potential to cause OATP-mediated drug-drug interactions.
Keywords: FDA Adverse Event Reporting System (FAERS); chloroquine (CQ); drug−drug interactions (DDIs); human sandwich-cultured hepatocytes (SCH); lysosomotropic drug; organic anion transporting polypeptide (OATP).
Figures


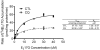
References
-
- Hagenbuch B, Meier PJ. Organic anion transporting polypeptides of the OATP/SLC21 family: phylogenetic classification as OATP/SLCO superfamily, new nomenclature and molecular/functional properties. Pfluegers Arch. 2004;447(5):653–665. - PubMed
-
- König J, Cui Y, Nies AT, Keppler D. A novel human organic anion transporting polypeptide localized to the basolateral hepatocyte membrane. Am J Physiol Gastrointest Liver Physiol. 2000;278(1):G156–G164. - PubMed
-
- König J. Uptake transporters of the human OATP family: molecular characteristics, substrates, their role in drug-drug interactions, and functional consequences of polymorphisms. Handb Exp Pharmacol. 2011;201:1–28. - PubMed
-
- Kiser JJ, Gerber JG, Predhomme JA, Wolfe P, Flynn DM, Hoody DW. Drug/Drug interaction between lopinavir/ritonavir and rosuvastatin in healthy volunteers. JAIDS, J Acquired Immune Defic Syndr. 2008;47(5):570–578. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

