1,25D3 prevents CD8(+)Tc2 skewing and asthma development through VDR binding changes to the Cyp11a1 promoter
- PMID: 26750596
- PMCID: PMC4712703
- DOI: 10.1038/ncomms10213
1,25D3 prevents CD8(+)Tc2 skewing and asthma development through VDR binding changes to the Cyp11a1 promoter
Abstract
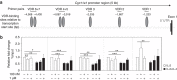
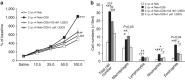
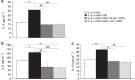
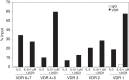
Effector CD8(+) T cells convert from IFN-γ(+) (Tc1) to IL-13(+) (Tc2) cells in the presence of IL-4. Underlying regulatory mechanisms are not fully defined. Here, we show that addition of 1,25D3, the active form of vitamin D3, during CD8(+) T-cell differentiation prevents IL-4-induced conversion to IL-13-producers. Transfer of 1,25D3-treated CD8(+) T cells into sensitized and challenged CD8(+)-deficient recipients fails to restore development of lung allergic responses. 1,25D3 alters vitamin D receptor (VDR) recruitment to the Cyp11a1 promoter in vitro and in vivo in the presence of IL-4. As a result, protein levels and enzymatic activity of CYP11A1, a steroidogenic enzyme regulating CD8(+) T-cell conversion, are decreased. An epistatic effect between CYP11A1 and VDR polymorphisms may contribute to the predisposition to childhood asthma. These data identify a role for 1,25D3 in the molecular programming of CD8(+) T-cell conversion to an IL-13-secreting phenotype through regulation of steroidogenesis, potentially governing asthma susceptibility.
Figures



Similar articles
-
CD8+ Tc2 cells: underappreciated contributors to severe asthma.Eur Respir Rev. 2019 Nov 20;28(154):190092. doi: 10.1183/16000617.0092-2019. Print 2019 Dec 31. Eur Respir Rev. 2019. PMID: 31748421 Free PMC article. Review.
-
Steroidogenic enzyme Cyp11a1 regulates Type 2 CD8+ T cell skewing in allergic lung disease.Proc Natl Acad Sci U S A. 2013 May 14;110(20):8152-7. doi: 10.1073/pnas.1216671110. Epub 2013 Apr 29. Proc Natl Acad Sci U S A. 2013. PMID: 23630275 Free PMC article.
-
Regulation of Th2 responses and allergic inflammation through bystander activation of CD8+ T lymphocytes in early life.J Immunol. 2010 Jul 15;185(2):884-91. doi: 10.4049/jimmunol.0903287. Epub 2010 Jun 18. J Immunol. 2010. PMID: 20562264
-
Increased IL-4- and IL-17-producing CD8+ cells are related to decreased CD39+CD4+Foxp3+ cells in allergic asthma.J Asthma. 2018 Jan;55(1):8-14. doi: 10.1080/02770903.2017.1310225. Epub 2017 May 23. J Asthma. 2018. PMID: 28346024
-
Effector CD8+ T cells mediate inflammation and airway hyper-responsiveness.Nat Med. 2004 Aug;10(8):865-9. doi: 10.1038/nm1081. Epub 2004 Jul 18. Nat Med. 2004. PMID: 15258576
Cited by
-
Vitamin D receptor gene polymorphisms in atopy.Immun Inflamm Dis. 2021 Dec;9(4):1153-1159. doi: 10.1002/iid3.487. Epub 2021 Aug 3. Immun Inflamm Dis. 2021. PMID: 34343413 Free PMC article. Review.
-
CD8+ Tc2 cells: underappreciated contributors to severe asthma.Eur Respir Rev. 2019 Nov 20;28(154):190092. doi: 10.1183/16000617.0092-2019. Print 2019 Dec 31. Eur Respir Rev. 2019. PMID: 31748421 Free PMC article. Review.
-
Autocrine vitamin D signaling switches off pro-inflammatory programs of TH1 cells.Nat Immunol. 2022 Jan;23(1):62-74. doi: 10.1038/s41590-021-01080-3. Epub 2021 Nov 11. Nat Immunol. 2022. PMID: 34764490 Free PMC article.
-
Nuclear Receptors in Asthma: Empowering Classical Molecules Against a Contemporary Ailment.Front Immunol. 2021 Jan 26;11:594433. doi: 10.3389/fimmu.2020.594433. eCollection 2020. Front Immunol. 2021. PMID: 33574813 Free PMC article. Review.
-
The Role of Vitamins in the Pathogenesis of Asthma.Int J Mol Sci. 2023 May 10;24(10):8574. doi: 10.3390/ijms24108574. Int J Mol Sci. 2023. PMID: 37239921 Free PMC article. Review.
References
-
- Isogai S. et al.. CD8+ alphabeta T cells can mediate late airway responses and airway eosinophilia in rats. J. Allergy Clin. Immunol. 114, 1345–1352 (2004). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

