Mycobacterium leprae-Infected Macrophages Preferentially Primed Regulatory T Cell Responses and Was Associated with Lepromatous Leprosy
- PMID: 26751388
- PMCID: PMC4713426
- DOI: 10.1371/journal.pntd.0004335
Mycobacterium leprae-Infected Macrophages Preferentially Primed Regulatory T Cell Responses and Was Associated with Lepromatous Leprosy
Abstract
Background: The persistence of Mycobacterium leprae (M. leprae) infection is largely dependent on the types of host immune responses being induced. Macrophage, a crucial modulator of innate and adaptive immune responses, could be directly infected by M. leprae. We therefore postulated that M. leprae-infected macrophages might have altered immune functions.
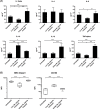
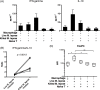
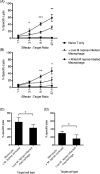
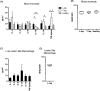
Methodology/principal findings: Here, we treated monocyte-derived macrophages with live or killed M. leprae, and examined their activation status and antigen presentation. We found that macrophages treated with live M. leprae showed committed M2-like function, with decreased interleukin 1 beta (IL-1beta), IL-6, tumor necrosis factor alpha (TNF-alpha) and MHC class II molecule expression and elevated IL-10 and CD163 expression. When incubating with naive T cells, macrophages treated with live M. leprae preferentially primed regulatory T (Treg) cell responses with elevated FoxP3 and IL-10 expression, while interferon gamma (IFN-gamma) expression and CD8+ T cell cytotoxicity were reduced. Chromium release assay also found that live M. leprae-treated macrophages were more resistant to CD8+ T cell-mediated cytotoxicity than sonicated M. leprae-treated monocytes. Ex vivo studies showed that the phenotype and function of monocytes and macrophages had clear differences between L-lep and T-lep patients, consistent with the in vitro findings.
Conclusions/significance: Together, our data demonstrate that M. leprae could utilize infected macrophages by two mechanisms: firstly, M. leprae-infected macrophages preferentially primed Treg but not Th1 or cytotoxic T cell responses; secondly, M. leprae-infected macrophages were more effective at evading CD8+ T cell-mediated cytotoxicity.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Analysis of cytokine production by Mycobacterium-reactive T cells. Failure to explain Mycobacterium leprae-specific nonresponsiveness of peripheral blood T cells from lepromatous leprosy patients.J Immunol. 1993 May 15;150(10):4641-51. J Immunol. 1993. PMID: 8482851
-
Long-term culture of multibacillary leprosy macrophages isolated from skin lesions: a new model to study Mycobacterium leprae-human cell interaction.Br J Dermatol. 2007 Aug;157(2):273-83. doi: 10.1111/j.1365-2133.2007.07992.x. Epub 2007 Jun 6. Br J Dermatol. 2007. PMID: 17553031
-
Differential development of CD4 and CD8 cytotoxic T cells (CTL) in PBMC across the leprosy spectrum; IL-6 with IFN-gamma or IL-2 generate CTL in multibacillary patients.Int J Lepr Other Mycobact Dis. 1997 Mar;65(1):45-55. Int J Lepr Other Mycobact Dis. 1997. PMID: 9207753
-
Mechanisms of Mycobacterium leprae-specific T-cell deficiency in lepromatous leprosy.Biochimie. 1988 Aug;70(8):1013-8. doi: 10.1016/0300-9084(88)90264-7. Biochimie. 1988. PMID: 3147697 Review.
-
HLA and leprosy in the pre and postgenomic eras.Hum Immunol. 2006 Jun;67(6):439-45. doi: 10.1016/j.humimm.2006.03.009. Epub 2006 Apr 3. Hum Immunol. 2006. PMID: 16728267 Review.
Cited by
-
Host Lipid Mediators in Leprosy: The Hypothesized Contributions to Pathogenesis.Front Immunol. 2018 Feb 2;9:134. doi: 10.3389/fimmu.2018.00134. eCollection 2018. Front Immunol. 2018. PMID: 29472920 Free PMC article. Review.
-
The MHC locus and genetic susceptibility to autoimmune and infectious diseases.Genome Biol. 2017 Apr 27;18(1):76. doi: 10.1186/s13059-017-1207-1. Genome Biol. 2017. PMID: 28449694 Free PMC article. Review.
-
Microbes, macrophages, and melanin: a unifying theory of disease as exemplified by cancer.Front Immunol. 2025 Feb 6;15:1493978. doi: 10.3389/fimmu.2024.1493978. eCollection 2024. Front Immunol. 2025. PMID: 39981299 Free PMC article. Review.
-
The cell fate regulator NUPR1 is induced by Mycobacterium leprae via type I interferon in human leprosy.PLoS Negl Trop Dis. 2019 Jul 25;13(7):e0007589. doi: 10.1371/journal.pntd.0007589. eCollection 2019 Jul. PLoS Negl Trop Dis. 2019. PMID: 31344041 Free PMC article.
-
Progress of the Art of Macrophage Polarization and Different Subtypes in Mycobacterial Infection.Front Immunol. 2021 Nov 9;12:752657. doi: 10.3389/fimmu.2021.752657. eCollection 2021. Front Immunol. 2021. PMID: 34899703 Free PMC article. Review.
References
-
- Walker SL, Lockwood DNJ. The clinical and immunological features of leprosy. Br Med Bull. 2006;77–78: 103–21. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

