Pinnisterols A-C, New 9,11-Secosterols from a Gorgonian Pinnigorgia sp
- PMID: 26751457
- PMCID: PMC4728509
- DOI: 10.3390/md14010012
Pinnisterols A-C, New 9,11-Secosterols from a Gorgonian Pinnigorgia sp
Abstract
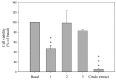
Three new 9,11-secosterols, pinnisterols A-C (1-3), were isolated from a gorgonian coral Pinnigorgia sp., collected off the waters of Taiwan. The structures of these compounds were elucidated on the basis of spectroscopic methods. The new sterols 1 and 3 displayed significant inhibitory effects on the generation of superoxide anions and the release of elastase by human neutrophils, and sterol 1 was found to show moderate cytotoxicity in hepatic stellate cells (HSCs).
Keywords: HSCs; Pinnigorgia; anti-inflammatory; cytotoxicity; elastase; gorgonian; secosterol; superoxide anion.
Figures
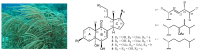


References
-
- Cheng S.-Y., Chen H.-P., Wang S.-K., Duh C.-Y. Three new 9,11-secosterols from the Formosan soft coral Sinularia leptoclados. Bull. Chem. Soc. Jpn. 2011;84:648–652. doi: 10.1246/bcsj.20110046. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

