Asymmetric Mach-Zehnder Interferometer Based Biosensors for Aflatoxin M1 Detection
- PMID: 26751486
- PMCID: PMC4810393
- DOI: 10.3390/bios6010001
Asymmetric Mach-Zehnder Interferometer Based Biosensors for Aflatoxin M1 Detection
Abstract
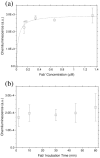
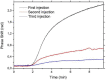
In this work, we present a study of Aflatoxin M1 detection by photonic biosensors based on Si₃N₄ Asymmetric Mach-Zehnder Interferometer (aMZI) functionalized with antibodies fragments (Fab'). We measured a best volumetric sensitivity of 10⁴ rad/RIU, leading to a Limit of Detection below 5 × 10(-7) RIU. On sensors functionalized with Fab', we performed specific and non-specific sensing measurements at various toxin concentrations. Reproducibility of the measurements and re-usability of the sensor were also investigated.
Keywords: Aflatoxin M1; Fab′; Mach–Zehnder interferometer; biosensor; limit of detection.
Figures




References
-
- Iqbal M., Gleeson M.A., Spaugh B., Tybor F., Gunn W.G., Hochberg M., Baehr-Jones T., Bailey R.C., Gunn L.C. Label-free biosensor arrays based on silicon ring resonators and high-speed optical scanning instrumentation. IEEE J. Sel. Top. Quantum Electron. 2010;16:654–661. doi: 10.1109/JSTQE.2009.2032510. - DOI
-
- Prieto F., Sepulveda B., Calle A., Llobera A., Domínguez C., Abad A., Montoya A., Lechuga L.M. An integrated optical interferometric nanodevice based on silicon technology for biosensor applications. Nanotechnology. 2003;14 doi: 10.1088/0957-4484/14/8/312. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

