Activation of the DNA Damage Response by RNA Viruses
- PMID: 26751489
- PMCID: PMC4808796
- DOI: 10.3390/biom6010002
Activation of the DNA Damage Response by RNA Viruses
Abstract
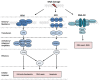
RNA viruses are a genetically diverse group of pathogens that are responsible for some of the most prevalent and lethal human diseases. Numerous viruses introduce DNA damage and genetic instability in host cells during their lifecycles and some species also manipulate components of the DNA damage response (DDR), a complex and sophisticated series of cellular pathways that have evolved to detect and repair DNA lesions. Activation and manipulation of the DDR by DNA viruses has been extensively studied. It is apparent, however, that many RNA viruses can also induce significant DNA damage, even in cases where viral replication takes place exclusively in the cytoplasm. DNA damage can contribute to the pathogenesis of RNA viruses through the triggering of apoptosis, stimulation of inflammatory immune responses and the introduction of deleterious mutations that can increase the risk of tumorigenesis. In addition, activation of DDR pathways can contribute positively to replication of viral RNA genomes. Elucidation of the interactions between RNA viruses and the DDR has provided important insights into modulation of host cell functions by these pathogens. This review summarises the current literature regarding activation and manipulation of the DDR by several medically important RNA viruses.
Keywords: DNA damage response; HCV; HIV-1; HTLV-1; IBV; Influenza A; RNA viruses; retroviruses.
Figures

Similar articles
-
Virus DNA Replication and the Host DNA Damage Response.Annu Rev Virol. 2018 Sep 29;5(1):141-164. doi: 10.1146/annurev-virology-092917-043534. Epub 2018 Jul 11. Annu Rev Virol. 2018. PMID: 29996066 Free PMC article. Review.
-
Modulation of DNA damage and repair pathways by human tumour viruses.Viruses. 2015 May 22;7(5):2542-91. doi: 10.3390/v7052542. Viruses. 2015. PMID: 26008701 Free PMC article. Review.
-
Viruses and the DNA Damage Response: Activation and Antagonism.Annu Rev Virol. 2014 Nov;1(1):605-25. doi: 10.1146/annurev-virology-031413-085548. Epub 2014 Jul 16. Annu Rev Virol. 2014. PMID: 26958736
-
Ubiquitination at the interface of tumor viruses and DNA damage responses.Curr Opin Virol. 2018 Oct;32:40-47. doi: 10.1016/j.coviro.2018.08.017. Epub 2018 Sep 24. Curr Opin Virol. 2018. PMID: 30261451 Free PMC article. Review.
-
Viral Modulation of the DNA Damage Response and Innate Immunity: Two Sides of the Same Coin.J Mol Biol. 2022 Mar 30;434(6):167327. doi: 10.1016/j.jmb.2021.167327. Epub 2021 Oct 22. J Mol Biol. 2022. PMID: 34695379 Free PMC article. Review.
Cited by
-
The consequences of viral infection on host DNA damage response: a focus on SARS-CoVs.J Genet Eng Biotechnol. 2022 Jul 13;20(1):104. doi: 10.1186/s43141-022-00388-3. J Genet Eng Biotechnol. 2022. PMID: 35829826 Free PMC article. Review.
-
Recombination between coronaviruses and synthetic RNAs and biorisk implications motivated by a SARS-CoV-2 FCS origin controversy.Front Bioeng Biotechnol. 2023 Aug 4;11:1209054. doi: 10.3389/fbioe.2023.1209054. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 37600318 Free PMC article.
-
Virus DNA Replication and the Host DNA Damage Response.Annu Rev Virol. 2018 Sep 29;5(1):141-164. doi: 10.1146/annurev-virology-092917-043534. Epub 2018 Jul 11. Annu Rev Virol. 2018. PMID: 29996066 Free PMC article. Review.
-
The impact of oxidative stress and the NRF2-KEAP1-ARE signaling pathway on anticancer drug resistance.Oncol Res. 2025 Jul 18;33(8):1819-1834. doi: 10.32604/or.2025.065755. eCollection 2025. Oncol Res. 2025. PMID: 40746900 Free PMC article. Review.
-
ZIKA virus elicits P53 activation and genotoxic stress in human neural progenitors similar to mutations involved in severe forms of genetic microcephaly.Cell Death Dis. 2016 Oct 27;7(10):e2440. doi: 10.1038/cddis.2016.266. Cell Death Dis. 2016. PMID: 27787521 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

