Targeting Stromal-Cancer Cell Crosstalk Networks in Ovarian Cancer Treatment
- PMID: 26751490
- PMCID: PMC4808797
- DOI: 10.3390/biom6010003
Targeting Stromal-Cancer Cell Crosstalk Networks in Ovarian Cancer Treatment
Abstract
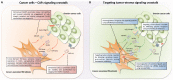
Ovarian cancer is a histologically, clinically, and molecularly diverse disease with a five-year survival rate of less than 30%. It has been estimated that approximately 21,980 new cases of epithelial ovarian cancer will be diagnosed and 14,270 deaths will occur in the United States in 2015, making it the most lethal gynecologic malignancy. Ovarian tumor tissue is composed of cancer cells and a collection of different stromal cells. There is increasing evidence that demonstrates that stromal involvement is important in ovarian cancer pathogenesis. Therefore, stroma-specific signaling pathways, stroma-derived factors, and genetic changes in the tumor stroma present unique opportunities for improving the diagnosis and treatment of ovarian cancer. Cancer-associated fibroblasts (CAFs) are one of the major components of the tumor stroma that have demonstrated supportive roles in tumor progression. In this review, we highlight various types of signaling crosstalk between ovarian cancer cells and stromal cells, particularly with CAFs. In addition to evaluating the importance of signaling crosstalk in ovarian cancer progression, we discuss approaches that can be used to target tumor-promoting signaling crosstalk and how these approaches can be translated into potential ovarian cancer treatment.
Keywords: cancer-associated fibroblasts; ovarian cancer; stromal-tumor crosstalk; tumor microenvironment.
Figures
Similar articles
-
Systematic Identification of Druggable Epithelial-Stromal Crosstalk Signaling Networks in Ovarian Cancer.J Natl Cancer Inst. 2019 Mar 1;111(3):272-282. doi: 10.1093/jnci/djy097. J Natl Cancer Inst. 2019. PMID: 29860390 Free PMC article.
-
Metformin Suppresses Tumor Progression by Inactivating Stromal Fibroblasts in Ovarian Cancer.Mol Cancer Ther. 2018 Jun;17(6):1291-1302. doi: 10.1158/1535-7163.MCT-17-0927. Epub 2018 Mar 15. Mol Cancer Ther. 2018. PMID: 29545331
-
The chemokine growth-regulated oncogene 1 (Gro-1) links RAS signaling to the senescence of stromal fibroblasts and ovarian tumorigenesis.Proc Natl Acad Sci U S A. 2006 Oct 31;103(44):16472-7. doi: 10.1073/pnas.0605752103. Epub 2006 Oct 23. Proc Natl Acad Sci U S A. 2006. PMID: 17060621 Free PMC article.
-
Ovarian cancer microenvironment: implications for cancer dissemination and chemoresistance acquisition.Cancer Metastasis Rev. 2014 Mar;33(1):17-39. doi: 10.1007/s10555-013-9456-2. Cancer Metastasis Rev. 2014. PMID: 24357056 Review.
-
A comprehensive overview of ovarian cancer stem cells: correlation with high recurrence rate, underlying mechanisms, and therapeutic opportunities.Mol Cancer. 2025 May 7;24(1):135. doi: 10.1186/s12943-025-02345-3. Mol Cancer. 2025. PMID: 40329326 Free PMC article. Review.
Cited by
-
PIM Kinases and Their Relevance to the PI3K/AKT/mTOR Pathway in the Regulation of Ovarian Cancer.Biomolecules. 2018 Feb 4;8(1):7. doi: 10.3390/biom8010007. Biomolecules. 2018. PMID: 29401696 Free PMC article. Review.
-
Identification of Copper Homeostasis-Related Gene Signature for Predicting Prognosis in Patients with Epithelial Ovarian Cancer.Cancer Inform. 2024 Aug 13;23:11769351241272400. doi: 10.1177/11769351241272400. eCollection 2024. Cancer Inform. 2024. PMID: 39139301 Free PMC article.
-
Prucalopride Inhibits Proliferation of Ovarian Cancer Cells via Phosphatidylinositol 3-Kinase (PI3K) Signaling Pathway.Med Sci Monit. 2018 Jun 17;24:4137-4145. doi: 10.12659/MSM.907853. Med Sci Monit. 2018. PMID: 29909423 Free PMC article.
-
Octreotide-Paclitaxel Conjugate Reverses Paclitaxel Resistance by p38 Mitogen-Activated Protein Kinase (MAPK) Signaling Pathway in A2780/Taxol Human Ovarian Cancer Cells.Med Sci Monit. 2020 Aug 23;26:e922612. doi: 10.12659/MSM.922612. Med Sci Monit. 2020. PMID: 32829374 Free PMC article.
-
The Role of Epithelial-to-Mesenchymal Plasticity in Ovarian Cancer Progression and Therapy Resistance.Cancers (Basel). 2019 Jun 17;11(6):838. doi: 10.3390/cancers11060838. Cancers (Basel). 2019. PMID: 31213009 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

