Tau Protein Hyperphosphorylation and Aggregation in Alzheimer's Disease and Other Tauopathies, and Possible Neuroprotective Strategies
- PMID: 26751493
- PMCID: PMC4808800
- DOI: 10.3390/biom6010006
Tau Protein Hyperphosphorylation and Aggregation in Alzheimer's Disease and Other Tauopathies, and Possible Neuroprotective Strategies
Abstract
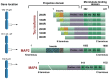
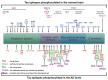
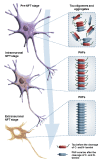
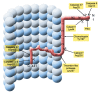
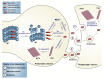
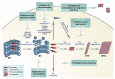
Abnormal deposition of misprocessed and aggregated proteins is a common final pathway of most neurodegenerative diseases, including Alzheimer's disease (AD). AD is characterized by the extraneuronal deposition of the amyloid β (Aβ) protein in the form of plaques and the intraneuronal aggregation of the microtubule-associated protein tau in the form of filaments. Based on the biochemically diverse range of pathological tau proteins, a number of approaches have been proposed to develop new potential therapeutics. Here we discuss some of the most promising ones: inhibition of tau phosphorylation, proteolysis and aggregation, promotion of intra- and extracellular tau clearance, and stabilization of microtubules. We also emphasize the need to achieve a full understanding of the biological roles and post-translational modifications of normal tau, as well as the molecular events responsible for selective neuronal vulnerability to tau pathology and its propagation. It is concluded that answering key questions on the relationship between Aβ and tau pathology should lead to a better understanding of the nature of secondary tauopathies, especially AD, and open new therapeutic targets and strategies.
Keywords: Alzheimer’s disease; amyloid β; microtubules; neurofibrillary degeneration; neuropathology; phosphorylation; protein aggregation; protein oligomerization; tau protein; tauopathies.
Figures


References
-
- Bielschowsky M. Die Silberimprägnation der Achsenzylinder. Neurol. Zentralb. (Leipzig) 1902;13:579–584. (In German)
-
- Alzheimer A. Uber eine eigenartige Erkrankung der Hirnrinde. Allg Zeits Psychiatry Psych. Med. 1907;64:146–148. (In German)
-
- Jucker M., Beyreuther K., Haass C., Nitsch R., Christen Y. Alzheimer: 100 Years and Beyond. Springer; Berlin, Germany: 2006.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

