Characteristics of Tau and Its Ligands in PET Imaging
- PMID: 26751494
- PMCID: PMC4808801
- DOI: 10.3390/biom6010007
Characteristics of Tau and Its Ligands in PET Imaging
Abstract
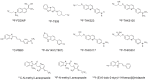
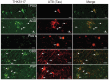
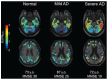
Tau deposition is one of the neuropathological hallmarks in Alzheimer's disease as well as in other neurodegenerative disorders called tauopathies. Recent efforts to develop selective tau radiopharmaceuticals have allowed the visualization of tau deposits in vivo. In vivo tau imaging allows the assessment of the regional distribution of tau deposits in a single human subject over time for determining the pathophysiology of tau accumulation in aging and neurodegenerative conditions as well as for application in drug discovery of anti-dementia drugs as surrogate markers. However, tau deposits show complicated characteristics because of different isoform composition, histopathology, and ultrastructure in various neurodegenerative conditions. In addition, since tau radiopharmaceuticals possess different chemotype classes, they may show different binding characteristics with heterogeneous tau deposits. In this review, we describe the characteristics of tau deposits and their ligands that have β-sheet binding properties, and the status of tau imaging in clinical studies.
Keywords: Alzheimer’s disease; positron emission tomography; radiotracer; tau deposits.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

