Functional Analysis of the Glucuronyltransferases GlcAT-P and GlcAT-S of Drosophila melanogaster: Distinct Activities towards the O-linked T-antigen
- PMID: 26751495
- PMCID: PMC4808802
- DOI: 10.3390/biom6010008
Functional Analysis of the Glucuronyltransferases GlcAT-P and GlcAT-S of Drosophila melanogaster: Distinct Activities towards the O-linked T-antigen
Abstract
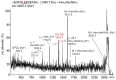
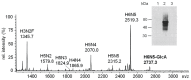
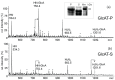
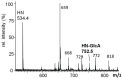
The Drosophila melanogaster glucuronyltransferases dGlcAT-S and dGlcAT-P were reported to be expressed ubiquitously and results of in vitro activity assays indicate a functional redundancy. We analyzed both transferases in vivo and in vitro and could show significant differences in their activity towards N-and O-glycoproteins in vivo. While GlcAT-P is able to use N-linked N-acetyllactosamine chains and the O-linked T-antigen as a substrate to form non-sulfated HNK1- (GlcAβ1-3Galβ1-4GlcNAcβ1-) and glucuronyl-T-antigens in vivo, GlcAT-S adds glucuronic acid only to N-linked chains, thereby synthesizing only the non-sulfated HNK1-antigen.
Keywords: Drosophila melanogaster; N-glycans; O-glycans; glucuronyltransferases; glycomics; mass spectrometry.
Figures

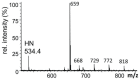
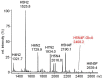
Similar articles
-
Identification and characterization of three Drosophila melanogaster glucuronyltransferases responsible for the synthesis of the conserved glycosaminoglycan-protein linkage region of proteoglycans. Two novel homologs exhibit broad specificity toward oligosaccharides from proteoglycans, glycoproteins, and glycosphingolipids.J Biol Chem. 2003 Mar 14;278(11):9116-24. doi: 10.1074/jbc.M209344200. Epub 2003 Jan 2. J Biol Chem. 2003. PMID: 12511570
-
Different acceptor specificities of two glucuronyltransferases involved in the biosynthesis of HNK-1 carbohydrate.Glycobiology. 2005 Feb;15(2):203-10. doi: 10.1093/glycob/cwi001. Epub 2004 Oct 6. Glycobiology. 2005. PMID: 15470230
-
Structural basis for acceptor substrate recognition of a human glucuronyltransferase, GlcAT-P, an enzyme critical in the biosynthesis of the carbohydrate epitope HNK-1.J Biol Chem. 2004 May 21;279(21):22693-703. doi: 10.1074/jbc.M400622200. Epub 2004 Mar 1. J Biol Chem. 2004. PMID: 14993226
-
The glycomics of glycan glucuronylation in Drosophila melanogaster.Methods Enzymol. 2010;480:297-321. doi: 10.1016/S0076-6879(10)80014-X. Methods Enzymol. 2010. PMID: 20816215 Review.
-
Enzyme assay of glucuronyltransferases for HNK-1 epitope (GlcAT-P, B3GAT1 and GlcAT-S, B3GAT2).2021 Sep 7 [updated 2022 Mar 14]. In: Nishihara S, Angata K, Aoki-Kinoshita KF, Hirabayashi J, editors. Glycoscience Protocols (GlycoPODv2) [Internet]. Saitama (JP): Japan Consortium for Glycobiology and Glycotechnology; 2021–. 2021 Sep 7 [updated 2022 Mar 14]. In: Nishihara S, Angata K, Aoki-Kinoshita KF, Hirabayashi J, editors. Glycoscience Protocols (GlycoPODv2) [Internet]. Saitama (JP): Japan Consortium for Glycobiology and Glycotechnology; 2021–. PMID: 37590727 Free Books & Documents. Review. No abstract available.
Cited by
-
Tissue-specific glycosylation in the honeybee: Analysis of the N-glycomes of Apis mellifera larvae and venom.Biochim Biophys Acta Gen Subj. 2019 Nov;1863(11):129409. doi: 10.1016/j.bbagen.2019.08.002. Epub 2019 Aug 6. Biochim Biophys Acta Gen Subj. 2019. PMID: 31398379 Free PMC article.
-
A conserved major facilitator superfamily member orchestrates a subset of O-glycosylation to aid macrophage tissue invasion.Elife. 2019 Mar 26;8:e41801. doi: 10.7554/eLife.41801. Elife. 2019. PMID: 30910009 Free PMC article.
-
Mucin-Type O-Glycosylation in the Drosophila Nervous System.Front Neuroanat. 2021 Oct 18;15:767126. doi: 10.3389/fnana.2021.767126. eCollection 2021. Front Neuroanat. 2021. PMID: 34733141 Free PMC article.
-
Glucuronylated core 1 glycans are required for precise localization of neuromuscular junctions and normal formation of basement membranes on Drosophila muscles.Dev Biol. 2018 Apr 15;436(2):108-124. doi: 10.1016/j.ydbio.2018.02.017. Epub 2018 Feb 27. Dev Biol. 2018. PMID: 29499182 Free PMC article.
-
Functional analysis of glycosylation using Drosophila melanogaster.Glycoconj J. 2020 Feb;37(1):1-14. doi: 10.1007/s10719-019-09892-0. Epub 2019 Nov 26. Glycoconj J. 2020. PMID: 31773367 Review.
References
-
- Aoki K., Porterfield M., Lee S., Dong B., Nguyen K., McGlamry K.H., Tiemeyer M. The diversity of O-linked glycans expressed during Drosophila melanogaster development reflects stage- and tissue-specific requirements for cell signaling. J. Biol. Chem. 2008;283:30385–30400. doi: 10.1074/jbc.M804925200. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

