A systematic review of the efficacy of live attenuated influenza vaccine upon revaccination of children
- PMID: 26751513
- PMCID: PMC4964816
- DOI: 10.1080/21645515.2015.1115164
A systematic review of the efficacy of live attenuated influenza vaccine upon revaccination of children
Abstract
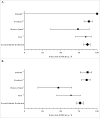
Four randomized, double-blind, placebo-controlled studies in 6090 children that investigated the efficacy of live attenuated influenza vaccine (LAIV) upon revaccination of children against laboratory-confirmed cases of influenza in consecutive seasons were reviewed. The efficacy in season 2 of LAIV administered over 2 consecutive seasons was 86.7% (95 % CI: 76.8%, 92.4%) against strains antigenically similar to those contained in the vaccine. The additional efficacy of LAIV administered in season 2 compared to LAIV recipients in season 1 only was 58.4% (28.3%, 75.9%). LAIV administered over 2 consecutive seasons also was more efficacious than was LAIV administered in season 2 only (relative efficacy: 53.9% [17.4%, 74.3%]). Residual efficacy of LAIV administered in season 1 only compared to placebo administered in two consecutive seasons was 56.4% (37.0%, 69.8%). This review did not find any evidence of decreasing efficacy of LAIV when administered during 2 consecutive seasons.
Keywords: children; efficacy; live attenuated influenza vaccine; repeat vaccination; revaccination.
Figures
Similar articles
-
Vaccines for preventing influenza in healthy children.Cochrane Database Syst Rev. 2018 Feb 1;2(2):CD004879. doi: 10.1002/14651858.CD004879.pub5. Cochrane Database Syst Rev. 2018. PMID: 29388195 Free PMC article.
-
Interactions between live attenuated influenza vaccine and nasopharyngeal microbiota among children aged 24-59 months in The Gambia: a phase 4, open-label, randomised controlled trial.Lancet Microbe. 2025 Mar;6(3):100971. doi: 10.1016/j.lanmic.2024.100971. Epub 2025 Jan 17. Lancet Microbe. 2025. PMID: 39832517 Clinical Trial.
-
Real-world effectiveness of live attenuated influenza vaccines (LAIV) and inactivated influenza vaccines (IIV) in children from 2003 to 2023: a systematic literature review and network meta-analysis.Expert Rev Vaccines. 2025 Dec;24(1):703-725. doi: 10.1080/14760584.2025.2536087. Epub 2025 Jul 30. Expert Rev Vaccines. 2025. PMID: 40697050
-
Vaccines for preventing influenza in healthy children.Cochrane Database Syst Rev. 2012 Aug 15;2012(8):CD004879. doi: 10.1002/14651858.CD004879.pub4. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2018 Feb 01;2:CD004879. doi: 10.1002/14651858.CD004879.pub5. PMID: 22895945 Free PMC article. Updated.
-
Influenza vaccine for chronic obstructive pulmonary disease (COPD).Cochrane Database Syst Rev. 2018 Jun 26;6(6):CD002733. doi: 10.1002/14651858.CD002733.pub3. Cochrane Database Syst Rev. 2018. PMID: 29943802 Free PMC article.
Cited by
-
Paediatric Virology and its interaction between basic science and clinical practice (Review).Int J Mol Med. 2018 Mar;41(3):1165-1176. doi: 10.3892/ijmm.2018.3364. Epub 2018 Jan 4. Int J Mol Med. 2018. PMID: 29328393 Free PMC article. Review.
-
Recombinant Measles AIK-C Vaccine Strain Expressing Influenza HA Protein.Vaccines (Basel). 2020 Mar 27;8(2):149. doi: 10.3390/vaccines8020149. Vaccines (Basel). 2020. PMID: 32230902 Free PMC article.
-
Association of Prior Vaccination With Influenza Vaccine Effectiveness in Children Receiving Live Attenuated or Inactivated Vaccine.JAMA Netw Open. 2018 Oct 5;1(6):e183742. doi: 10.1001/jamanetworkopen.2018.3742. JAMA Netw Open. 2018. PMID: 30646262 Free PMC article.
-
A live attenuated virus-based intranasal COVID-19 vaccine provides rapid, prolonged, and broad protection against SARS-CoV-2.Sci Bull (Beijing). 2022 Jul 15;67(13):1372-1387. doi: 10.1016/j.scib.2022.05.018. Epub 2022 May 26. Sci Bull (Beijing). 2022. PMID: 35637645 Free PMC article.
-
[Health Technology Assessment (HTA) of the introduction of influenza vaccination for Italian children with Fluenz Tetra®].J Prev Med Hyg. 2021 Sep 10;62(2 Suppl 1):E1-E118. doi: 10.15167/2421-4248/jpmh2021.62.2s1. eCollection 2021 Jun. J Prev Med Hyg. 2021. PMID: 34909481 Free PMC article. Italian. No abstract available.
References
-
- Heikkinen T, Heinonen S. Effectiveness and safety of influenza vaccination in children: European perspective. Vaccine 2011; 29:7529–34. PMID:21820481 - PubMed
-
- Ohmit SE, Thompson MG, Petrie JG, Thaker SN, Jackson ML, Belongia EA, Zimmerman RK, Gaglani M, Lamerato L, Spencer SM, et al.. Influenza vaccine effectiveness in the 2011–2012 season: protection against each circulating virus and the effect of prior vaccination on estimates. Clin Infect Dis 2014; 58:319–27. PMID:24235265; http://dx.doi.org/10.1093/cid/cit736 - DOI - PMC - PubMed
-
- Skowronski DM, Janjua NZ, Sabaiduc S, De Serres G, Winter AL, Gubbay JB, Dickinson JA, Fonseca K, Charest H, Bastien N, et al.. Influenza A/Subtype and B/Lineage effectiveness estimates for the 2011–2012 trivalent vaccine: cross-season and cross-lineage protection with unchanged vaccine. J Infect Dis 2014; 210:126–37. - PubMed
-
- Bracco Neto H, Farhat CK, Tregnaghi MW, Madhi SA, Razmpour A, Palladino G, Small MG, Gruber WC, Forrest BD. Efficacy and safety of 1 and 2 doses of live attenuated influenza vaccine in vaccine-naive children. Pediatr Infect Dis J 2009; 28:365–71. PMID:19395948 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
