Association of CMV, HBV, or HCV co-infection with vaccine response in adults with well-controlled HIV infection
- PMID: 26751638
- PMCID: PMC4963073
- DOI: 10.1080/21645515.2015.1121336
Association of CMV, HBV, or HCV co-infection with vaccine response in adults with well-controlled HIV infection
Abstract
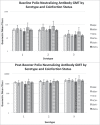
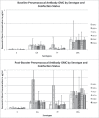
Even after CD4 count recovery on antiretroviral therapy, HIV infection is associated with decreased response to most vaccines compared to the general population. Chronic infections with viruses such as cytomegalovirus (CMV), hepatitis B virus (HBV), and hepatitis C virus (HCV), which are more prevalent in HIV-infected populations, have been linked to immune dysfunction and decreased vaccine response in the general population. However, whether co-infection with these other viruses contributes to the decreased vaccine response seen in adults with well-controlled HIV infection is unknown. We conducted a secondary analysis of data and serum from adults with well-controlled HIV infection from an inactivated polio vaccine trial (224 subjects) and a pneumococcal conjugate vaccine study (128 subjects). We evaluated the association of CMV, HBV, or HCV co-infection with post-vaccination antibody levels using both univariate and multivariate analyses, controlling for factors such as age, race, CD4 count, comorbidities, smoking status, and baseline antibody levels. Ninety-three percent, 7%, and 14% of subjects were co-infected with CMV, HBV, and HCV respectively. On both univariate and multivariate analysis, neither CMV nor HCV co-infection were significantly associated with post-vaccination antibody levels to either vaccine. HBV co-infection was significantly associated with post-vaccination antibody concentrations for pneumococcal serotype 7F on univariate analysis and 6A on multivariate analysis, but the association was with higher antibody concentrations. In conclusion, co-infection with CMV, HBV, or HCV does not appear to contribute to the decreased vaccine response seen in adults with well-controlled HIV infection.
Keywords: CMV; HBV; HCV; HIV; vaccine response.
Figures
References
-
- Heffernan RT, Barrett NL, Gallagher KM, Hadler JL, Harrison LH, Reingold AL, Khoshnood K, Holford TR, Schuchat A. Declining incidence of invasive Streptococcus pneumoniae infections among persons with AIDS in an era of highly active antiretroviral therapy, 1995–2000. J Infect Dis 2005; 191:2038-45; PMID:15897989; http://dx.doi.org/10.1086/430356 - DOI - PubMed
-
- Cohen C, Simonsen L, Sample J, Kang JW, Miller M, Madhi SA, Campsmith M, Viboud C. Influenza-related mortality among adults aged 25–54 years with AIDS in South Africa and the United States of America. Clin Infect Dis 2012; 55:996-1003; PMID:22715173; http://dx.doi.org/10.1093/cid/cis549 - DOI - PMC - PubMed
-
- Miller L, Arakaki L, Ramautar A, Bodach S, Braunstein SL, Kennedy J, Steiner-Sichel L, Ngai S, Shepard C, Weiss D. Elevated risk for invasive meningococcal disease among persons with HIV. Ann Intern Med 2014; 160:30-7; PMID:24166695; http://dx.doi.org/10.7326/P14-9011 - DOI - PubMed
-
- Laurence JC. Hepatitis A and B immunizations of individuals infected with human immunodeficiency virus. Am J Med 2005; 118 Suppl 10A:75S-83S; PMID:16271546; http://dx.doi.org/10.1016/j.amjmed.2005.07.024 - DOI - PubMed
-
- Crum-Cianflone NF, Eberly LE, Duplessis C, Maguire J, Ganesan A, Faix D, Defang G, Bai Y, Iverson E, Lalani T, et al.. Immunogenicity of a monovalent 2009 influenza A (H1N1) vaccine in an immunocompromised population: a prospective study comparing HIV-infected adults with HIV-uninfected adults. Clin Infect Dis 2011; 52:138-46; PMID:21148532; http://dx.doi.org/10.1093/cid/ciq019 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
