Inhibition of Aerobic Glycolysis Attenuates Disease Progression in Polycystic Kidney Disease
- PMID: 26752072
- PMCID: PMC4708993
- DOI: 10.1371/journal.pone.0146654
Inhibition of Aerobic Glycolysis Attenuates Disease Progression in Polycystic Kidney Disease
Abstract
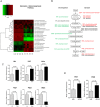
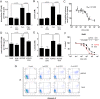
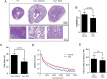
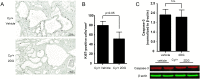
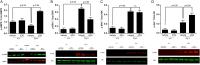
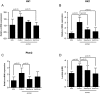
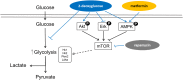
Dysregulated signaling cascades alter energy metabolism and promote cell proliferation and cyst expansion in polycystic kidney disease (PKD). Here we tested whether metabolic reprogramming towards aerobic glycolysis ("Warburg effect") plays a pathogenic role in male heterozygous Han:SPRD rats (Cy/+), a chronic progressive model of PKD. Using microarray analysis and qPCR, we found an upregulation of genes involved in glycolysis (Hk1, Hk2, Ldha) and a downregulation of genes involved in gluconeogenesis (G6pc, Lbp1) in cystic kidneys of Cy/+ rats compared with wild-type (+/+) rats. We then tested the effect of inhibiting glycolysis with 2-deoxyglucose (2DG) on renal functional loss and cyst progression in 5-week-old male Cy/+ rats. Treatment with 2DG (500 mg/kg/day) for 5 weeks resulted in significantly lower kidney weights (-27%) and 2-kidney/total-body-weight ratios (-20%) and decreased renal cyst index (-48%) compared with vehicle treatment. Cy/+ rats treated with 2DG also showed higher clearances of creatinine (1.98±0.67 vs 1.41±0.37 ml/min), BUN (0.69±0.26 vs 0.40±0.10 ml/min) and uric acid (0.38±0.20 vs 0.21±0.10 ml/min), and reduced albuminuria. Immunoblotting analysis of kidney tissues harvested from 2DG-treated Cy/+ rats showed increased phosphorylation of AMPK-α, a negative regulator of mTOR, and restoration of ERK signaling. Assessment of Ki-67 staining indicated that 2DG limits cyst progression through inhibition of epithelial cell proliferation. Taken together, our results show that targeting the glycolytic pathway may represent a promising therapeutic strategy to control cyst growth in PKD.
Conflict of interest statement
Figures

References
-
- Torres VE, Harris PC, Pirson Y (2007) Autosomal dominant polycystic kidney disease. Lancet 369: 1287–1301. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

