Activation of raphe nuclei triggers rapid and distinct effects on parallel olfactory bulb output channels
- PMID: 26752161
- PMCID: PMC4948943
- DOI: 10.1038/nn.4219
Activation of raphe nuclei triggers rapid and distinct effects on parallel olfactory bulb output channels
Abstract
The serotonergic raphe nuclei are involved in regulating brain states over timescales of minutes and hours. We examined more rapid effects of raphe activation on two classes of principal neurons in the mouse olfactory bulb, mitral and tufted cells, which send olfactory information to distinct targets. Brief stimulation of the raphe nuclei led to excitation of tufted cells at rest and potentiation of their odor responses. While mitral cells at rest were also excited by raphe activation, their odor responses were bidirectionally modulated, leading to improved pattern separation of odors. In vitro whole-cell recordings revealed that specific optogenetic activation of raphe axons affected bulbar neurons through dual release of serotonin and glutamate. Therefore, the raphe nuclei, in addition to their role in neuromodulation of brain states, are also involved in fast, sub-second top-down modulation similar to cortical feedback. This modulation can selectively and differentially sensitize or decorrelate distinct output channels.
Figures
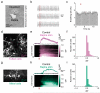
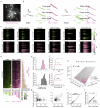

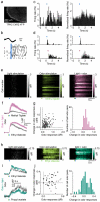

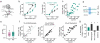


Comment in
-
Rapid control of olfaction.Nat Neurosci. 2016 Feb;19(2):181. doi: 10.1038/nn0216-181. Nat Neurosci. 2016. PMID: 26814586 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

