Age-dependent impact of CaV 3.2 T-type calcium channel deletion on myogenic tone and flow-mediated vasodilatation in small arteries
- PMID: 26752249
- PMCID: PMC5063926
- DOI: 10.1113/JP271470
Age-dependent impact of CaV 3.2 T-type calcium channel deletion on myogenic tone and flow-mediated vasodilatation in small arteries
Abstract
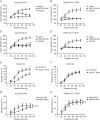
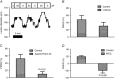
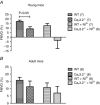
Key points: Blood pressure and flow exert mechanical forces on the walls of small arteries, which are detected by the endothelial and smooth muscle cells, and lead to regulation of the diameter (basal tone) of an artery. CaV 3.2 T-type calcium channels are expressed in the wall of small arteries, although their function remains poorly understood because of the low specificity of T-type blockers. We used mice deficient in CaV 3.2 channels to study their role in pressure- and flow-dependent tone regulation and the possible impact of ageing on this role. In young mice, CaV 3.2 channels oppose pressure-induced vasoconstriction and participate in endothelium-dependent, flow-mediated dilatation. These effects were not seen in mature adult mice. The results of the present study demonstrate an age-dependent impact of CaV 3.2 T-type calcium channel deletion in rodents and suggest that the loss of CaV 3.2 channel function leads to more constricted arteries, which is a risk factor for cardiovascular disease.
Abstract: The myogenic response and flow-mediated vasodilatation are important regulators of local blood perfusion and total peripheral resistance, and are known to entail a calcium influx into vascular smooth muscle cells (VSMCs) and endothelial cells (ECs), respectively. CaV 3.2 T-type calcium channels are expressed in both VSMCs and ECs of small arteries. The T-type channels are important drug targets but, as a result of the lack of specific antagonists, our understanding of the role of CaV 3.2 channels in vasomotor tone at various ages is scarce. We evaluated the myogenic response, flow-mediated vasodilatation, structural remodelling and mRNA + protein expression in small mesenteric arteries from CaV 3.2 knockout (CaV 3.2KO) vs. wild-type mice at a young vs. mature adult age. In young mice only, deletion of CaV 3.2 led to an enhanced myogenic response and a ∼50% reduction of flow-mediated vasodilatation. Ni2+ had both CaV 3.2-dependent and independent effects. No changes in mRNA expression of several important K+ and Ca2+ channel genes were induced by CaV 3.2KO However, the expression of the other T-type channel isoform (CaV 3.1) was reduced at the mRNA and protein level in mature adult compared to young wild-type arteries. The results of the present study demonstrate the important roles of the CaV 3.2 T-type calcium channels in myogenic tone and flow-mediated vasodilatation that disappear with ageing. Because increased arterial tone is a risk factor for cardiovascular disease, we conclude that CaV 3.2 channels, by modulating pressure- and flow-mediated vasomotor responses to prevent excess arterial tone, protect against cardiovascular disease.
© 2016 The Authors. The Journal of Physiology © 2016 The Physiological Society.
Figures





Similar articles
-
Myogenic tone is impaired at low arterial pressure in mice deficient in the low-voltage-activated CaV 3.1 T-type Ca(2+) channel.Acta Physiol (Oxf). 2013 Apr;207(4):709-20. doi: 10.1111/apha.12066. Epub 2013 Feb 18. Acta Physiol (Oxf). 2013. PMID: 23356724
-
Differential targeting and signalling of voltage-gated T-type Cav 3.2 and L-type Cav 1.2 channels to ryanodine receptors in mesenteric arteries.J Physiol. 2018 Oct;596(20):4863-4877. doi: 10.1113/JP276923. Epub 2018 Sep 15. J Physiol. 2018. PMID: 30146760 Free PMC article.
-
Genetic ablation of CaV3.2 channels enhances the arterial myogenic response by modulating the RyR-BKCa axis.Arterioscler Thromb Vasc Biol. 2015 Aug;35(8):1843-51. doi: 10.1161/ATVBAHA.115.305736. Epub 2015 Jun 11. Arterioscler Thromb Vasc Biol. 2015. PMID: 26069238 Free PMC article.
-
Ion channels and the regulation of myogenic tone in peripheral arterioles.Curr Top Membr. 2020;85:19-58. doi: 10.1016/bs.ctm.2020.01.002. Epub 2020 Feb 25. Curr Top Membr. 2020. PMID: 32402640 Free PMC article. Review.
-
Calcium-Dependent Ion Channels and the Regulation of Arteriolar Myogenic Tone.Front Physiol. 2021 Nov 8;12:770450. doi: 10.3389/fphys.2021.770450. eCollection 2021. Front Physiol. 2021. PMID: 34819877 Free PMC article. Review.
Cited by
-
Regular Black Bean Consumption Is Necessary to Sustain Improvements in Small-Artery Vascular Compliance in the Spontaneously Hypertensive Rat.Nutrients. 2020 Mar 3;12(3):685. doi: 10.3390/nu12030685. Nutrients. 2020. PMID: 32138293 Free PMC article.
-
Role of age, Rho-kinase 2 expression, and G protein-mediated signaling in the myogenic response in mouse small mesenteric arteries.Physiol Rep. 2018 Sep;6(17):e13863. doi: 10.14814/phy2.13863. Physiol Rep. 2018. PMID: 30198176 Free PMC article.
-
Voltage-dependent inward currents in smooth muscle cells of skeletal muscle arterioles.PLoS One. 2018 Apr 25;13(4):e0194980. doi: 10.1371/journal.pone.0194980. eCollection 2018. PLoS One. 2018. PMID: 29694371 Free PMC article.
-
Network medicine framework identified drug-repurposing opportunities of pharmaco-active compounds of Angelica acutiloba (Siebold & Zucc.) Kitag. for skin aging.Aging (Albany NY). 2023 Jun 12;15(11):5144-5163. doi: 10.18632/aging.204789. Epub 2023 Jun 12. Aging (Albany NY). 2023. PMID: 37310405 Free PMC article.
-
Tumour-specific amplitude-modulated radiofrequency electromagnetic fields induce differentiation of hepatocellular carcinoma via targeting Cav3.2 T-type voltage-gated calcium channels and Ca2+ influx.EBioMedicine. 2019 Jun;44:209-224. doi: 10.1016/j.ebiom.2019.05.034. Epub 2019 May 31. EBioMedicine. 2019. PMID: 31160272 Free PMC article.
References
-
- Ando J & Yamamoto K (2013). Flow detection and calcium signalling in vascular endothelial cells. Cardiovasc Res 99, 260–268. - PubMed
-
- Ball CJ, Wilson DP, Turner SP, Saint DA & Beltrame JF (2009). Heterogeneity of L‐ and T‐channels in the vasculature: rationale for the efficacy of combined L‐ and T‐blockade. Hypertension 53, 654–660. - PubMed
-
- Bhagyalakshmi A & Frangos JA (1989). Mechanism of shear‐induced prostacyclin production in endothelial cells. Biochem Biophys Res Commun 158, 31–37. - PubMed
-
- Björling K, Morita H, Olsen MF, Prodan A, Hansen PB, Lory P, Holstein‐Rathlou NH & Jensen LJ (2013). Myogenic tone is impaired at low arterial pressure in mice deficient in the low‐voltage‐activated CaV 3.1 T‐type Ca(2+) channel. Acta Physiol (Oxf) 207, 709–720. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

