Killer lymphocytes use granulysin, perforin and granzymes to kill intracellular parasites
- PMID: 26752517
- PMCID: PMC7325279
- DOI: 10.1038/nm.4023
Killer lymphocytes use granulysin, perforin and granzymes to kill intracellular parasites
Abstract
Protozoan infections are a serious global health problem. Natural killer (NK) cells and cytolytic T lymphocytes (CTLs) eliminate pathogen-infected cells by releasing cytolytic granule contents--granzyme (Gzm) proteases and the pore-forming perforin (PFN)--into the infected cell. However, these cytotoxic molecules do not kill intracellular parasites. CD8(+) CTLs protect against parasite infections in mice primarily by secreting interferon (IFN)-γ. However, human, but not rodent, cytotoxic granules contain the antimicrobial peptide granulysin (GNLY), which selectively destroys cholesterol-poor microbial membranes, and GNLY, PFN and Gzms rapidly kill intracellular bacteria. Here we show that GNLY delivers Gzms into three protozoan parasites (Trypanosoma cruzi, Toxoplasma gondii and Leishmania major), in which the Gzms generate superoxide and inactivate oxidative defense enzymes to kill the parasite. PFN delivers GNLY and Gzms into infected cells, and GNLY then delivers Gzms to the intracellular parasites. Killer cell-mediated parasite death, which we term 'microbe-programmed cell death' or 'microptosis', is caspase independent but resembles mammalian apoptosis, causing mitochondrial swelling, transmembrane potential dissipation, membrane blebbing, phosphatidylserine exposure, DNA damage and chromatin condensation. GNLY-transgenic mice are protected against infection by T. cruzi and T. gondii, and survive infections that are lethal to wild-type mice. Thus, GNLY-, PFN- and Gzm-mediated elimination of intracellular protozoan parasites is an unappreciated immune defense mechanism.
Conflict of interest statement
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Figures
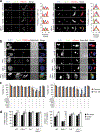

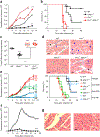

References
-
- Junqueira C et al. The endless race between Trypanosoma cruzi and host immunity: lessons for and beyond Chagas disease. Expert Rev. Mol. Med 12, e29 (2010). - PubMed
-
- Rodrigues MM et al. Importance of CD8 T cell–mediated immune response during intracellular parasitic infections and its implications for the development of effective vaccines. An. Acad. Bras. Cienc 75, 443–468 (2003). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

