CXCL13 drives spinal astrocyte activation and neuropathic pain via CXCR5
- PMID: 26752644
- PMCID: PMC4731172
- DOI: 10.1172/JCI81950
CXCL13 drives spinal astrocyte activation and neuropathic pain via CXCR5
Abstract
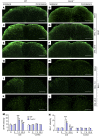
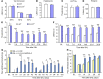
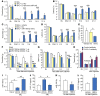
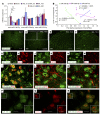
Recent studies have implicated chemokines in microglial activation and pathogenesis of neuropathic pain. C-X-C motif chemokine 13 (CXCL13) is a B lymphocyte chemoattractant that activates CXCR5. Using the spinal nerve ligation (SNL) model of neuropathic pain, we found that CXCL13 was persistently upregulated in spinal cord neurons after SNL, resulting in spinal astrocyte activation via CXCR5 in mice. shRNA-mediated inhibition of CXCL13 in the spinal cord persistently attenuated SNL-induced neuropathic pain. Interestingly, CXCL13 expression was suppressed by miR-186-5p, a microRNA that colocalized with CXCL13 and was downregulated after SNL. Spinal overexpression of miR-186-5p decreased CXCL13 expression, alleviating neuropathic pain. Furthermore, SNL induced CXCR5 expression in spinal astrocytes, and neuropathic pain was abrogated in Cxcr5-/- mice. CXCR5 expression induced by SNL was required for the SNL-induced activation of spinal astrocytes and microglia. Intrathecal injection of CXCL13 was sufficient to induce pain hypersensitivity and astrocyte activation via CXCR5 and ERK. Finally, intrathecal injection of CXCL13-activated astrocytes induced mechanical allodynia in naive mice. Collectively, our findings reveal a neuronal/astrocytic interaction in the spinal cord by which neuronally produced CXCL13 activates astrocytes via CXCR5 to facilitate neuropathic pain. Thus, miR-186-5p and CXCL13/CXCR5-mediated astrocyte signaling may be suitable therapeutic targets for neuropathic pain.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

