Rho-A prenylation and signaling link epithelial homeostasis to intestinal inflammation
- PMID: 26752649
- PMCID: PMC4731169
- DOI: 10.1172/JCI80997
Rho-A prenylation and signaling link epithelial homeostasis to intestinal inflammation
Abstract
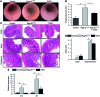
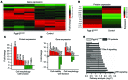
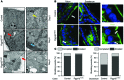
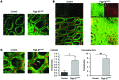
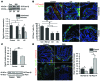
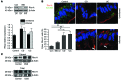
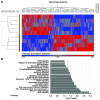
Although defects in intestinal barrier function are a key pathogenic factor in patients with inflammatory bowel diseases (IBDs), the molecular pathways driving disease-specific alterations of intestinal epithelial cells (IECs) are largely unknown. Here, we addressed this issue by characterizing the transcriptome of IECs from IBD patients using a genome-wide approach. We observed disease-specific alterations in IECs with markedly impaired Rho-A signaling in active IBD patients. Localization of epithelial Rho-A was shifted to the cytosol in IBDs, and inflammation was associated with suppressed Rho-A activation due to reduced expression of the Rho-A prenylation enzyme geranylgeranyltransferase-I (GGTase-I). Functionally, we found that mice with conditional loss of Rhoa or the gene encoding GGTase-I, Pggt1b, in IECs exhibit spontaneous chronic intestinal inflammation with accumulation of granulocytes and CD4+ T cells. This phenotype was associated with cytoskeleton rearrangement and aberrant cell shedding, ultimately leading to loss of epithelial integrity and subsequent inflammation. These findings uncover deficient prenylation of Rho-A as a key player in the pathogenesis of IBDs. As therapeutic triggering of Rho-A signaling suppressed intestinal inflammation in mice with GGTase-I-deficient IECs, our findings suggest new avenues for treatment of epithelial injury and mucosal inflammation in IBD patients.
Figures



Similar articles
-
Epithelial RAC1-dependent cytoskeleton dynamics controls cell mechanics, cell shedding and barrier integrity in intestinal inflammation.Gut. 2023 Feb;72(2):275-294. doi: 10.1136/gutjnl-2021-325520. Epub 2022 Mar 3. Gut. 2023. PMID: 35241625 Free PMC article.
-
Inhibiting PGGT1B Disrupts Function of RHOA, Resulting in T-cell Expression of Integrin α4β7 and Development of Colitis in Mice.Gastroenterology. 2019 Nov;157(5):1293-1309. doi: 10.1053/j.gastro.2019.07.007. Epub 2019 Jul 11. Gastroenterology. 2019. PMID: 31302143
-
Intestinal epithelial cells from inflammatory bowel disease patients preferentially stimulate CD4+ T cells to proliferate and secrete interferon-gamma.Am J Physiol Gastrointest Liver Physiol. 2007 Jun;292(6):G1630-40. doi: 10.1152/ajpgi.00294.2006. Epub 2007 Mar 8. Am J Physiol Gastrointest Liver Physiol. 2007. PMID: 17347451
-
Dysregulation of Intestinal Epithelial Cell RIPK Pathways Promotes Chronic Inflammation in the IBD Gut.Front Immunol. 2019 May 20;10:1094. doi: 10.3389/fimmu.2019.01094. eCollection 2019. Front Immunol. 2019. PMID: 31164887 Free PMC article. Review.
-
Pleiotropic functions of TNF-α in the regulation of the intestinal epithelial response to inflammation.Int Immunol. 2014 Sep;26(9):509-15. doi: 10.1093/intimm/dxu051. Epub 2014 May 12. Int Immunol. 2014. PMID: 24821262 Review.
Cited by
-
Epithelial RAC1-dependent cytoskeleton dynamics controls cell mechanics, cell shedding and barrier integrity in intestinal inflammation.Gut. 2023 Feb;72(2):275-294. doi: 10.1136/gutjnl-2021-325520. Epub 2022 Mar 3. Gut. 2023. PMID: 35241625 Free PMC article.
-
IL-20 controls resolution of experimental colitis by regulating epithelial IFN/STAT2 signalling.Gut. 2024 Jan 5;73(2):282-297. doi: 10.1136/gutjnl-2023-329628. Gut. 2024. PMID: 37884352 Free PMC article.
-
The complex interplay among hepatocytes and immune cells at the crossroad between inflammation and cholesterol metabolism in hyperglycemia.Endocrine. 2017 Oct;58(1):21-23. doi: 10.1007/s12020-017-1254-0. Epub 2017 Feb 8. Endocrine. 2017. PMID: 28181143 Review.
-
Wall of Resilience: How the Intestinal Epithelium Prevents Inflammatory Onslaught in the Gut.Cell Mol Gastroenterol Hepatol. 2025;19(2):101423. doi: 10.1016/j.jcmgh.2024.101423. Epub 2024 Oct 24. Cell Mol Gastroenterol Hepatol. 2025. PMID: 39461590 Free PMC article. Review.
-
Epithelial RAC1 niches in IBD: from barrier integrity to cytoskeletal plasticity.Gut. 2023 Feb;72(2):219-220. doi: 10.1136/gutjnl-2022-327089. Epub 2022 Apr 11. Gut. 2023. PMID: 35410889 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

