Essential Contribution of CD4+ T Cells to Antigen-Induced Nasal Hyperresponsiveness in Experimental Allergic Rhinitis
- PMID: 26752722
- PMCID: PMC4709066
- DOI: 10.1371/journal.pone.0146686
Essential Contribution of CD4+ T Cells to Antigen-Induced Nasal Hyperresponsiveness in Experimental Allergic Rhinitis
Expression of concern in
-
Expression of Concern: Essential Contribution of CD4+ T Cells to Antigen-Induced Nasal Hyperresponsiveness in Experimental Allergic Rhinitis.PLoS One. 2023 Jan 11;18(1):e0279797. doi: 10.1371/journal.pone.0279797. eCollection 2023. PLoS One. 2023. PMID: 36630363 Free PMC article. No abstract available.
Abstract
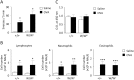
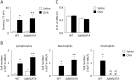
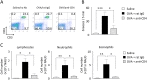
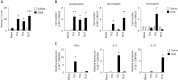
Nasal hyperresponsiveness (NHR) is a characteristic feature of allergic rhinitis (AR); however, the pathogenesis of NHR is not fully understood. In this study, during the establishment of an experimental AR model using ovalbumin-immunized and -challenged mice, augmentation of the sneezing reaction in response to nonspecific proteins as well as a chemical stimulant was detected. Whether NHR is independent of mast cells and eosinophils was determined by using mast cell- and eosinophil-deficient mice. NHR was suppressed by treatment with anti-CD4 antibody, suggesting the pivotal contribution of CD4+ T cells. Furthermore, antigen challenge to mice to which in vitro-differentiated Th1, Th2, and Th17 cells but not naïve CD4+ T cells had been adoptively transferred led to the development of equivalent NHR. Since antigen-specific IgE and IgG were not produced in these mice and since antigen-specific IgE-transgenic mice did not develop NHR even upon antigen challenge, humoral immunity would be dispensable for NHR. CD4+ T cells play a crucial role in the pathogenesis of AR via induction of NHR, independent of IgE-, mast cell-, and eosinophil-mediated responses.
Conflict of interest statement
Figures



Similar articles
-
Effects of anti-allergic drugs on T cell-mediated nasal hyperresponsiveness in a murine model of allergic rhinitis.Allergol Int. 2018 Sep;67S:S25-S31. doi: 10.1016/j.alit.2018.05.002. Epub 2018 Jun 15. Allergol Int. 2018. PMID: 29910099
-
T Cell-Mediated Nasal Hyperresponsiveness in Allergic Rhinitis.Biol Pharm Bull. 2020;43(1):36-40. doi: 10.1248/bpb.b18-01021. Biol Pharm Bull. 2020. PMID: 31902929 Review.
-
Allergen endotoxins induce T-cell-dependent and non-IgE-mediated nasal hypersensitivity in mice.J Allergy Clin Immunol. 2017 Jan;139(1):258-268.e10. doi: 10.1016/j.jaci.2016.03.023. Epub 2016 Jun 7. J Allergy Clin Immunol. 2017. PMID: 27287257
-
Listeriolysin O derived from Listeria monocytogenes inhibits the effector phase of an experimental allergic rhinitis induced by ovalbumin in mice.Clin Exp Immunol. 2006 Jun;144(3):475-84. doi: 10.1111/j.1365-2249.2006.03092.x. Clin Exp Immunol. 2006. PMID: 16734617 Free PMC article.
-
Role of IgE in the development of allergic airway inflammation and airway hyperresponsiveness--a murine model.Allergy. 1999 Apr;54(4):297-305. doi: 10.1034/j.1398-9995.1999.00085.x. Allergy. 1999. PMID: 10371087 Review.
Cited by
-
Suppressive Effect of Lactococcus lactis subsp. cremoris YRC3780 on a Murine Model of Japanese Cedar Pollinosis.Pathogens. 2022 Nov 14;11(11):1347. doi: 10.3390/pathogens11111347. Pathogens. 2022. PMID: 36422599 Free PMC article.
-
Suppressive effect of dexamethasone on murine Th9 cell-mediated nasal eosinophilic inflammation.Asia Pac Allergy. 2021 Jul 7;11(3):e25. doi: 10.5415/apallergy.2021.11.e25. eCollection 2021 Jul. Asia Pac Allergy. 2021. PMID: 34386401 Free PMC article.
-
Effects of anticholinergic agent on miRNA profiles and transcriptomes in a murine model of allergic rhinitis.Mol Med Rep. 2017 Nov;16(5):6558-6569. doi: 10.3892/mmr.2017.7411. Epub 2017 Aug 31. Mol Med Rep. 2017. PMID: 28901404 Free PMC article.
-
Hyper-reactive cloned mice generated by direct nuclear transfer of antigen-specific CD4+ T cells.EMBO Rep. 2017 Jun;18(6):885-893. doi: 10.15252/embr.201643321. Epub 2017 May 2. EMBO Rep. 2017. PMID: 28468955 Free PMC article.
-
Role of CD4+ T Cells in Allergic Airway Diseases: Learning from Murine Models.Int J Mol Sci. 2020 Oct 11;21(20):7480. doi: 10.3390/ijms21207480. Int J Mol Sci. 2020. PMID: 33050549 Free PMC article. Review.
References
-
- Baraniuk JN (2001) Mechanisms of allergic rhinitis. Curr Allergy Asthma Rep 1: 207–217. - PubMed
-
- Parikh SA, Cho SH, Oh CK (2003) Preformed enzymes in mast cell granules and their potential role in allergic rhinitis. Curr Allergy Asthma Rep 3: 266–272. - PubMed
-
- Sin B, Togias A (2011) Pathophysiology of allergic and nonallergic rhinitis. Proc Am, Thorac Soc 8: 106–114. - PubMed
-
- Kanthawatana S, Maturim W, Fooanant S, Manorot M, Trakultivakorn M (1997) Evaluation of threshold criteria for the nasal histamine challenge test in perennial allergic rhinitis. Asian Pac J Allergy Immunol 15: 65–69. - PubMed
-
- Sanico AM, Koliatsos VE, Stanisz AM, Bienenstock J, Togias A (1999) Neural hyperresponsiveness and nerve growth factor in allergic rhinitis. Int Arch Allergy Immunol 118: 154–158. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

