Malaria transmission dynamics surrounding the first nationwide long-lasting insecticidal net distribution in Papua New Guinea
- PMID: 26753618
- PMCID: PMC4709896
- DOI: 10.1186/s12936-015-1067-7
Malaria transmission dynamics surrounding the first nationwide long-lasting insecticidal net distribution in Papua New Guinea
Abstract
Background: The major malaria vectors of Papua New Guinea exhibit heterogeneities in distribution, biting behaviour and malaria infection levels. Long-lasting, insecticide-treated nets (LLINs), distributed as part of the National Malaria Control Programme, are the primary intervention targeting malaria transmission. This study evaluated the impact of LLINs on anopheline density, species composition, feeding behaviour, and malaria transmission.
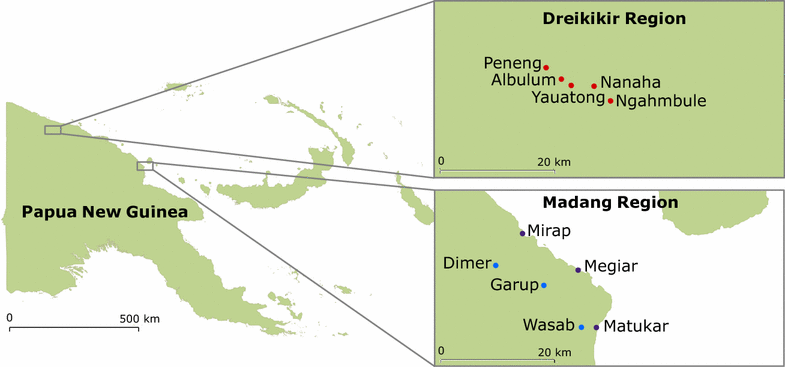
Methods: Mosquitoes were collected by human landing catch in 11 villages from East Sepik Province and Madang Province. Mosquitoes were collected for 3 years (1 year before distribution and 2 years after), and assayed to determine mosquito species and Plasmodium spp. infection prevalence. The influence of weather conditions and the presence of people and animals on biting density was determined. Determinants of biting density and sporozoite prevalence were analysed by generalized estimating equations (GEE).
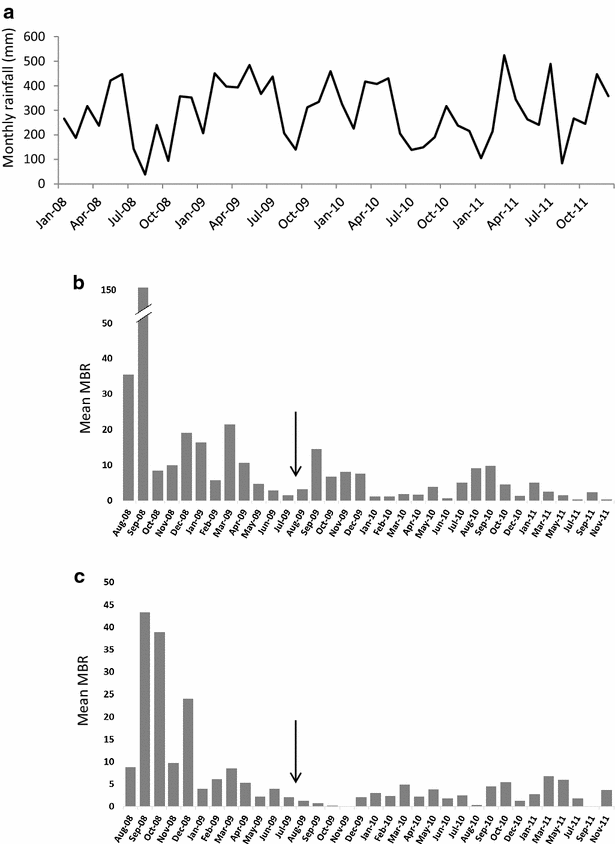
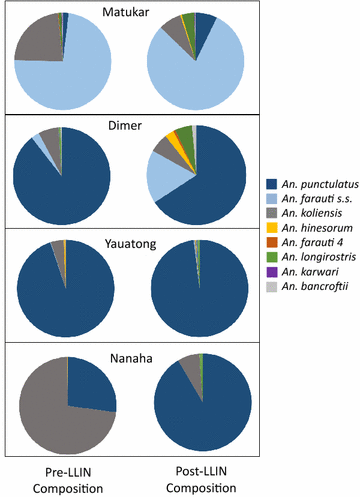
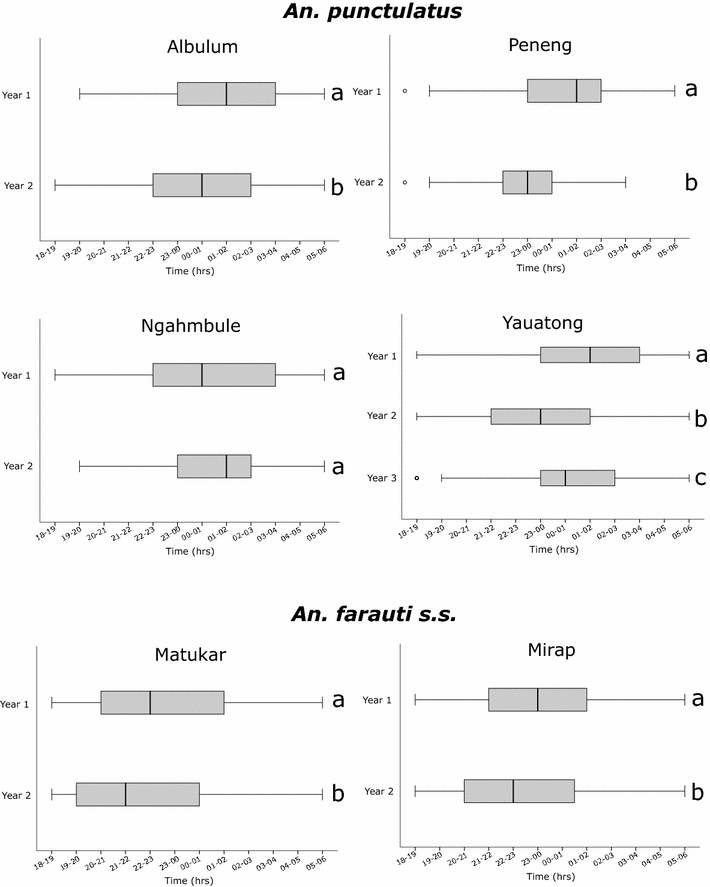
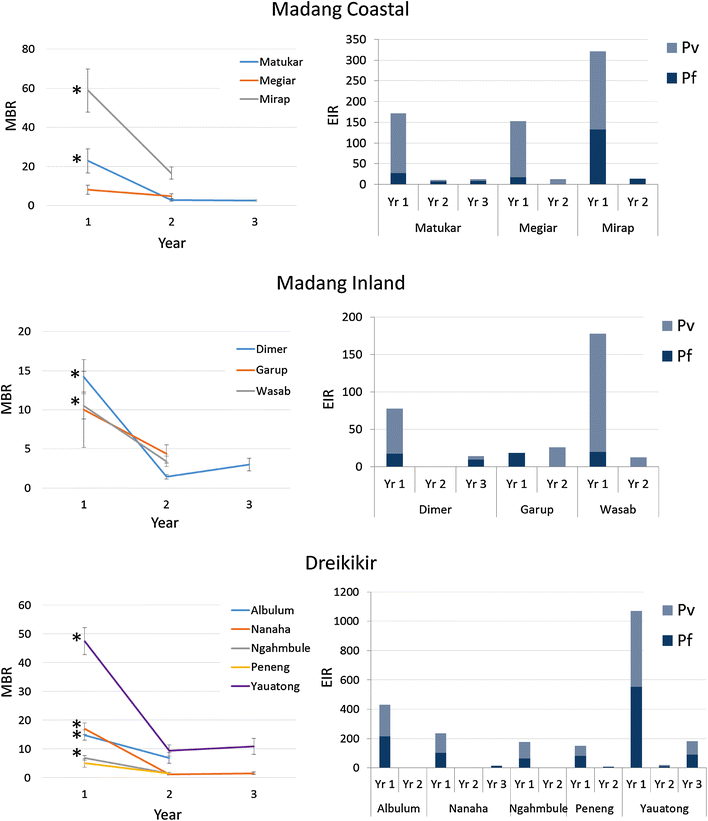
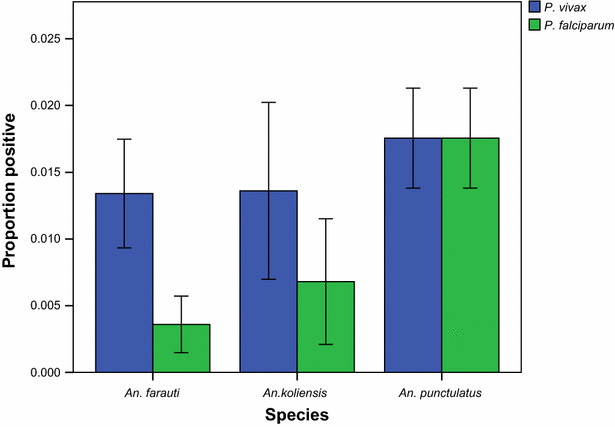
Results: Mosquito biting rates and entomological inoculation rates decreased significantly after the distribution. Plasmodium falciparum and P. vivax sporozoite prevalence decreased in year 2, but increased in year 3, suggesting the likelihood of resurgence in transmission if low biting rates are not maintained. An earlier shift in the median biting time of Anopheles punctulatus and An. farauti s.s. was observed. However, this was not accompanied by an increase in the proportion of infective bites occurring before 2200 hours. A change in species composition was observed, which resulted in dominance of An. punctulatus in Dreikikir region, but a decrease in An. punctulatus in the Madang region. When controlling for village and study year, An. farauti s.s., An. koliensis and An. punctulatus were equally likely to carry P. vivax sporozoites. However, An. punctulatus was significantly more likely than An. farauti s.s. (OR 0.14; p = 0.007) or An. koliensis (OR 0.27; p < 0.001) to carry P. falciparum sporozoites.
Conclusions: LLINs had a significant impact on malaria transmission, despite exophagic and crepuscular feeding behaviours of dominant vectors. Changes in species composition and feeding behaviour were observed, but their epidemiological significance will depend on their durability over time.
Figures
Similar articles
-
Vector composition, abundance, biting patterns and malaria transmission intensity in Madang, Papua New Guinea: assessment after 7 years of an LLIN-based malaria control programme.Malar J. 2022 Jan 5;21(1):7. doi: 10.1186/s12936-021-04030-4. Malar J. 2022. PMID: 34983530 Free PMC article.
-
Human malaria transmission studies in the Anopheles punctulatus complex in Papua New Guinea: sporozoite rates, inoculation rates, and sporozoite densities.Am J Trop Med Hyg. 1988 Aug;39(2):135-44. doi: 10.4269/ajtmh.1988.39.135. Am J Trop Med Hyg. 1988. PMID: 3044151
-
Changes in malaria burden and transmission in sentinel sites after the roll-out of long-lasting insecticidal nets in Papua New Guinea.Parasit Vectors. 2016 Jun 14;9(1):340. doi: 10.1186/s13071-016-1635-x. Parasit Vectors. 2016. PMID: 27301964 Free PMC article.
-
Current vector control challenges in the fight against malaria.Acta Trop. 2017 Oct;174:91-96. doi: 10.1016/j.actatropica.2017.06.028. Epub 2017 Jul 3. Acta Trop. 2017. PMID: 28684267 Review.
-
Malaria entomological profile in Tanzania from 1950 to 2010: a review of mosquito distribution, vectorial capacity and insecticide resistance.Tanzan J Health Res. 2011 Dec;13(5 Suppl 1):319-31. Tanzan J Health Res. 2011. PMID: 26591987 Review.
Cited by
-
Comparison of Different Mosquito Traps for Zoonotic Arbovirus Vectors in Papua New Guinea.Am J Trop Med Hyg. 2022 Jan 17;106(3):823-827. doi: 10.4269/ajtmh.21-0640. Am J Trop Med Hyg. 2022. PMID: 35026726 Free PMC article.
-
Mathematical models of Plasmodium vivax transmission: A scoping review.PLoS Comput Biol. 2024 Mar 14;20(3):e1011931. doi: 10.1371/journal.pcbi.1011931. eCollection 2024 Mar. PLoS Comput Biol. 2024. PMID: 38483975 Free PMC article.
-
Repeated mosquito net distributions, improved treatment, and trends in malaria cases in sentinel health facilities in Papua New Guinea.Malar J. 2019 Nov 12;18(1):364. doi: 10.1186/s12936-019-2993-6. Malar J. 2019. PMID: 31718659 Free PMC article.
-
Vector composition, abundance, biting patterns and malaria transmission intensity in Madang, Papua New Guinea: assessment after 7 years of an LLIN-based malaria control programme.Malar J. 2022 Jan 5;21(1):7. doi: 10.1186/s12936-021-04030-4. Malar J. 2022. PMID: 34983530 Free PMC article.
-
The epidemiology of Plasmodium falciparum and Plasmodium vivax in East Sepik Province, Papua New Guinea, pre- and post-implementation of national malaria control efforts.Malar J. 2020 Jun 5;19(1):198. doi: 10.1186/s12936-020-03265-x. Malar J. 2020. PMID: 32503607 Free PMC article.
References
-
- Mehlotra RK, Gray LR, Blood-Zikursh MJ, Kloos Z, Henry-Halldin CN, Tisch DJ, Thomsen E, Reimer L, Kastens W, Baea M, Baea K, Baisor M, Tarongka N, Kazura JW, Zimmerman PA. Short report: molecular-based assay for simultaneous detection of four Plasmodium spp. and Wuchereria bancrofti infections. Am J Trop Med Hyg. 2010;82:1030–1033. doi: 10.4269/ajtmh.2010.09-0665. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

