FBXO32, encoding a member of the SCF complex, is mutated in dilated cardiomyopathy
- PMID: 26753747
- PMCID: PMC4707779
- DOI: 10.1186/s13059-015-0861-4
FBXO32, encoding a member of the SCF complex, is mutated in dilated cardiomyopathy
Abstract
Background: Dilated cardiomyopathy (DCM) is a common form of cardiomyopathy causing systolic dysfunction and heart failure. Rare variants in more than 30 genes, mostly encoding sarcomeric proteins and proteins of the cytoskeleton, have been implicated in familial DCM to date. Yet, the majority of variants causing DCM remain to be identified. The goal of the study is to identify novel mutations causing familial dilated cardiomyopathy.
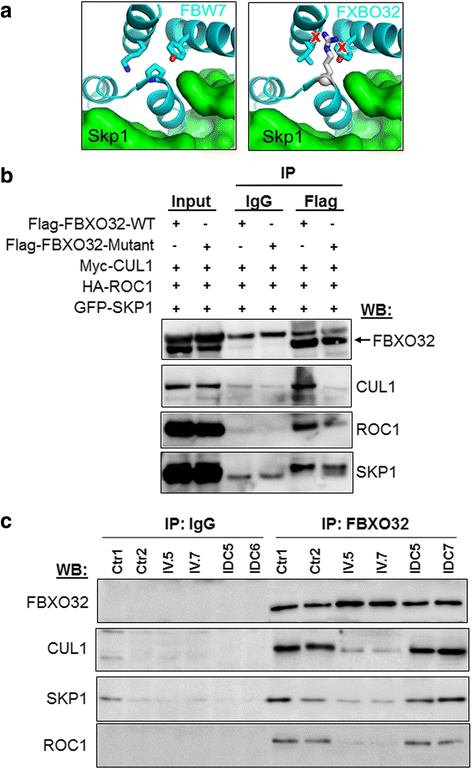
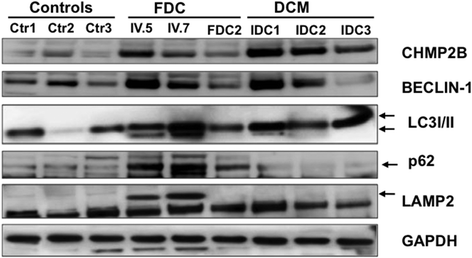
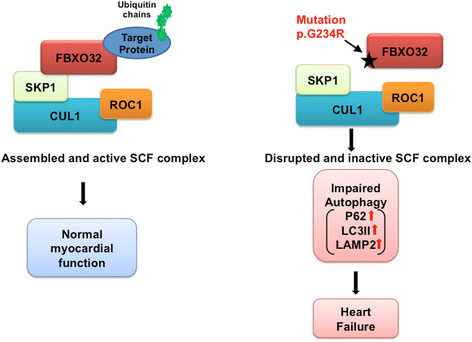
Results: We identify FBXO32 (ATROGIN 1), a member of the F-Box protein family, as a novel DCM-causing locus. The missense mutation affects a highly conserved amino acid and is predicted to severely impair binding to SCF proteins. This is validated by co-immunoprecipitation experiments from cells expressing the mutant protein and from human heart tissue from two of the affected patients. We also demonstrate that the hearts of the patients with the FBXO32 mutation show accumulation of selected proteins regulating autophagy.
Conclusion: Our results indicate that abnormal SCF activity with subsequent impairment of the autophagic flux due to a novel FBXO32 mutation is implicated in the pathogenesis of DCM.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

