The challenge of evolving stable polyploidy: could an increase in "crossover interference distance" play a central role?
- PMID: 26753761
- PMCID: PMC4830878
- DOI: 10.1007/s00412-015-0571-4
The challenge of evolving stable polyploidy: could an increase in "crossover interference distance" play a central role?
Abstract
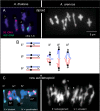
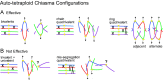
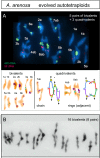
Whole genome duplication is a prominent feature of many highly evolved organisms, especially plants. When duplications occur within species, they yield genomes comprising multiple identical or very similar copies of each chromosome ("autopolyploids"). Such genomes face special challenges during meiosis, the specialized cellular program that underlies gamete formation for sexual reproduction. Comparisons between newly formed (neo)-autotetraploids and fully evolved autotetraploids suggest that these challenges are solved by specific restrictions on the positions of crossover recombination events and, thus, the positions of chiasmata, which govern the segregation of homologs at the first meiotic division. We propose that a critical feature in the evolution of these more effective chiasma patterns is an increase in the effective distance of meiotic crossover interference, which plays a central role in crossover positioning. We discuss the findings in several organisms, including the recent identification of relevant genes in Arabidopsis arenosa, that support this hypothesis.
Keywords: Chiasmata; Crossover interference; Homologous chromosomes; Meiosis; Polyploidy; Recombination.
Figures



References
-
- Albini SM, Jones GH. Synaptonemal complex spreading in Allium cepa and A. fistulosum. I. The initiation and sequence of pairing. Chromosoma. 1987;95:324–338. doi: 10.1007/BF00293179. - DOI
-
- Astaurov BL. Experimental polyploidy in animals. Annu Rev Genet. 1969;3:99–126. doi: 10.1146/annurev.ge.03.120169.000531. - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

