Simeprevir with pegylated interferon alfa 2a plus ribavirin for treatment of hepatitis C virus genotype 1 in patients with HIV: a meta-analysis and historical comparison
- PMID: 26753774
- PMCID: PMC4709957
- DOI: 10.1186/s12879-015-1311-3
Simeprevir with pegylated interferon alfa 2a plus ribavirin for treatment of hepatitis C virus genotype 1 in patients with HIV: a meta-analysis and historical comparison
Abstract
Background: About one third of patients infected with human immunodeficiency virus (HIV) also have chronic hepatitis due to hepatitis C virus (HCV). HCV therapy with simeprevir, pegylated interferon alfa (PegIFNα) and ribavirin (RBV) have been shown to be superior to PegIFNα + RBV alone in non-HIV patients, but no randomized trials in patients with HCV genotype 1 (HCV-1)/HIV coinfection are available.
Methods: This was a historical comparison of study C212 (simeprevir + PegIFNα-2a + RBV in patients with HCV-1/HIV coinfection) with studies in which HCV-1/HIV coinfected patients were treated with PegIFNα-2a + RBV alone. A systematic literature search was performed to identify eligible studies. Efficacy and safety results of PegIFNα-2a + RBV studies were combined in random- and fixed-effects inverse-variance weighted meta-analyses of proportions using the Freeman-Tukey double arcsin transformation method, and compared with the results of study C212.
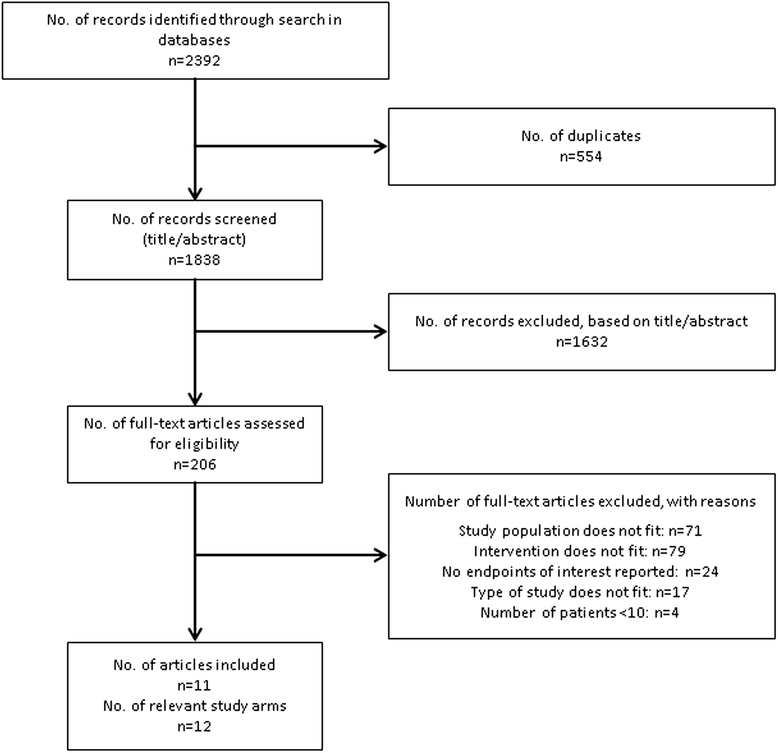
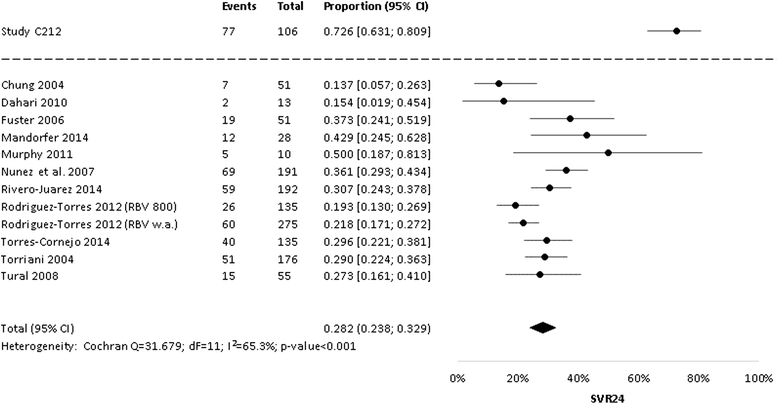
Results: The literature search revealed a total of 2392 records, with 206 articles selected for full-text review. Finally, 11 relevant articles reporting on 12 relevant study groups were included. Results on sustained virologic response 24 weeks after end of treatment (SVR24) were available from all 12 study groups. Pooled SVR24 for PegIFNα-2a + RBV from the random-effects meta-analysis was 28.2% (95% CI 23.8% to 32.9%). The comparison between study C212 (SVR24 = 72.6%; 95% CI 63.1% to 80.9%) revealed substantial superiority of simeprevir + PegIFNα-2a + RBV compared to PegIFNα-2a + RBV alone, with an absolute risk difference of 45% (95% CI 34 to 55). This finding was robust in a sensitivity analysis that only included historical studies with a planned treatment duration of at least 48 weeks and the same RBV dose as in study C212. No increases in the frequency of important adverse event categories including anemia were identified, but these analyses were limited by the low number of studies.
Conclusion: This historical comparison provides first systematic evidence for the superiority of simeprevir + PegIFNα-2a + RBV compared to PegIFNα-2a + RBV in patients with HCV-1/HIV coinfection. Given the limitations of the historical comparison for safety endpoints, additional data on the comparative safety of simeprevir in patients with HCV-1/HIV coinfection would be desirable.
Trial registration: Identifier for study TMC435-TiDP16-C212 (ClinicalTrials.gov): NCT01479868.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

