CD146/MCAM defines functionality of human bone marrow stromal stem cell populations
- PMID: 26753846
- PMCID: PMC4710006
- DOI: 10.1186/s13287-015-0266-z
CD146/MCAM defines functionality of human bone marrow stromal stem cell populations
Abstract
Background: Identification of surface markers for prospective isolation of functionally homogenous populations of human skeletal (stromal, mesenchymal) stem cells (hMSCs) is highly relevant for cell therapy protocols. Thus, we examined the possible use of CD146 to subtype a heterogeneous hMSC population.
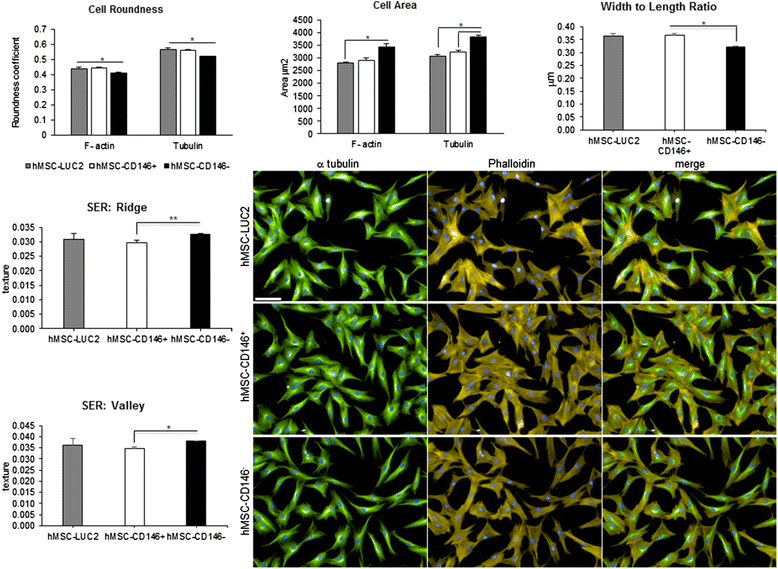
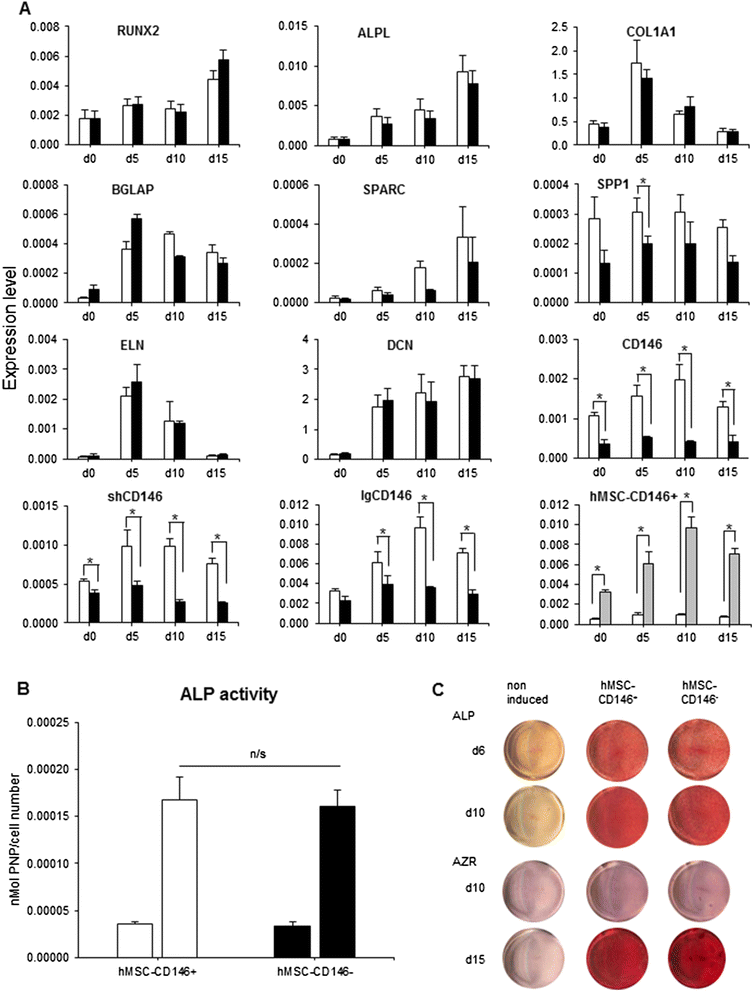
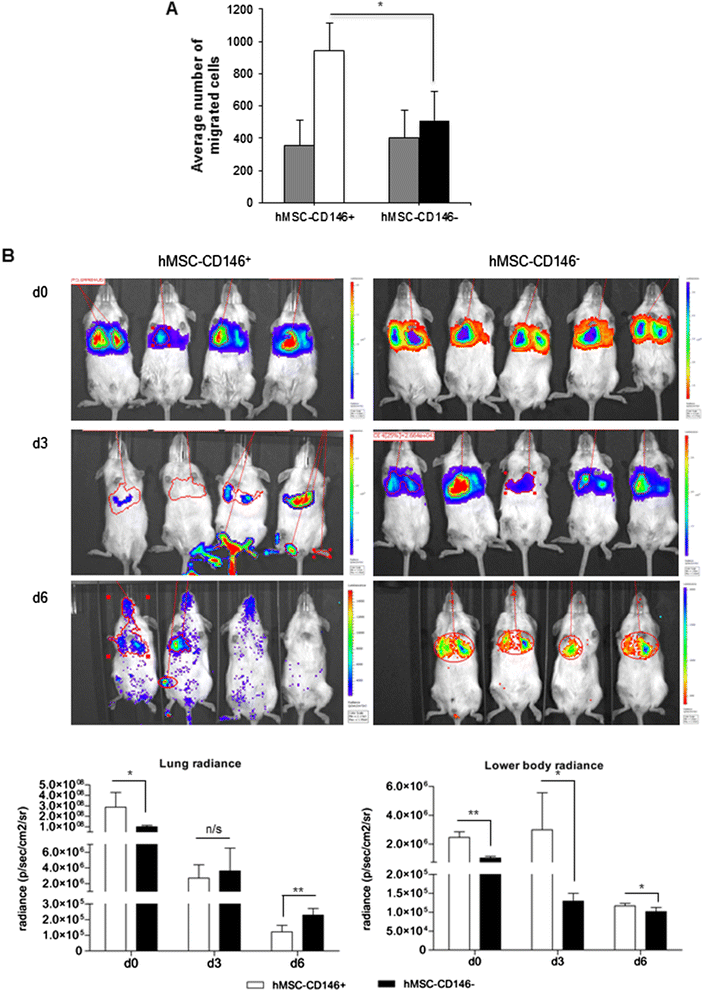
Methods: Using flow cytometry and cell sorting, we isolated two distinct hMSC-CD146(+) and hMSC-CD146(-) cell populations from the telomerized human bone marrow-derived stromal cell line (hMSC-TERT). Cells were examined for differences in their size, shape and texture by using high-content analysis and additionally for their ability to differentiate toward osteogenesis in vitro and form bone in vivo, and their migrational ability in vivo and in vitro was investigated.
Results: In vitro, the two cell populations exhibited similar growth rate and differentiation capacity to osteoblasts and adipocytes on the basis of gene expression and protein production of lineage-specific markers. In vivo, hMSC-CD146(+) and hMSC-CD146(-) cells formed bone and bone marrow organ when implanted subcutaneously in immune-deficient mice. Bone was enriched in hMSC-CD146(-) cells (12.6 % versus 8.1 %) and bone marrow elements enriched in implants containing hMSC-CD146(+) cells (0.5 % versus 0.05 %). hMSC-CD146(+) cells exhibited greater chemotactic attraction in a transwell migration assay and, when injected intravenously into immune-deficient mice following closed femoral fracture, exhibited wider tissue distribution and significantly increased migration ability as demonstrated by bioluminescence imaging.
Conclusion: Our studies demonstrate that CD146 defines a subpopulation of hMSCs capable of bone formation and in vivo trans-endothelial migration and thus represents a population of hMSCs suitable for use in clinical protocols of bone tissue regeneration.
Figures


Similar articles
-
Association between in vivo bone formation and ex vivo migratory capacity of human bone marrow stromal cells.Stem Cell Res Ther. 2015 Oct 8;6:196. doi: 10.1186/s13287-015-0188-9. Stem Cell Res Ther. 2015. PMID: 26450135 Free PMC article.
-
hMSC-Derived VEGF Release Triggers the Chemoattraction of Alveolar Osteoblasts.Stem Cells. 2015 Oct;33(10):3114-24. doi: 10.1002/stem.2119. Epub 2015 Aug 12. Stem Cells. 2015. PMID: 26235535
-
Regulation of human skeletal stem cells differentiation by Dlk1/Pref-1.J Bone Miner Res. 2004 May;19(5):841-52. doi: 10.1359/JBMR.040118. Epub 2004 Jan 19. J Bone Miner Res. 2004. PMID: 15068508
-
Regeneration of cartilage and bone by defined subsets of mesenchymal stromal cells--potential and pitfalls.Adv Drug Deliv Rev. 2011 Apr 30;63(4-5):342-51. doi: 10.1016/j.addr.2010.12.004. Epub 2010 Dec 22. Adv Drug Deliv Rev. 2011. PMID: 21184789 Review.
-
Human stromal (mesenchymal) stem cells: basic biology and current clinical use for tissue regeneration.Ann Saudi Med. 2012 Jan-Feb;32(1):68-77. doi: 10.5144/0256-4947.2012.68. Ann Saudi Med. 2012. PMID: 22156642 Free PMC article. Review.
Cited by
-
Fibroblasts direct differentiation of human breast epithelial progenitors.Breast Cancer Res. 2020 Sep 29;22(1):102. doi: 10.1186/s13058-020-01344-0. Breast Cancer Res. 2020. PMID: 32993755 Free PMC article.
-
Expression of CD146 and Regenerative Cytokines by Human Placenta-Derived Mesenchymal Stromal Cells upon Expansion in Different GMP-Compliant Media.Stem Cells Int. 2021 Apr 2;2021:6662201. doi: 10.1155/2021/6662201. eCollection 2021. Stem Cells Int. 2021. PMID: 33868409 Free PMC article.
-
Molecular and Functional Phenotypes of Human Bone Marrow-Derived Mesenchymal Stromal Cells Depend on Harvesting Techniques.Int J Mol Sci. 2020 Jun 19;21(12):4382. doi: 10.3390/ijms21124382. Int J Mol Sci. 2020. PMID: 32575596 Free PMC article.
-
The Bone Marrow-Derived Stromal Cells: Commitment and Regulation of Adipogenesis.Front Endocrinol (Lausanne). 2016 Sep 21;7:127. doi: 10.3389/fendo.2016.00127. eCollection 2016. Front Endocrinol (Lausanne). 2016. PMID: 27708616 Free PMC article. Review.
-
Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Hold Lower Heterogeneity and Great Promise in Biological Research and Clinical Applications.Front Cell Dev Biol. 2021 Sep 30;9:716907. doi: 10.3389/fcell.2021.716907. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34660579 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

