The involvement of patient organisations in rare disease research: a mixed methods study in Australia
- PMID: 26754025
- PMCID: PMC4709899
- DOI: 10.1186/s13023-016-0382-6
The involvement of patient organisations in rare disease research: a mixed methods study in Australia
Abstract
Background: We report here selected findings from a mixed-methods study investigating the role of Australian rare disease patient organisations (RDPOs) in research. Despite there being many examples of RDPOs that have initiated and supported significant scientific advances, there is little information - and none at all in Australia - about RDPOs generally, and their research-related goals, activities, and experiences. This information is a pre-requisite for understanding what RDPOs bring to research and how their involvement could be strengthened.
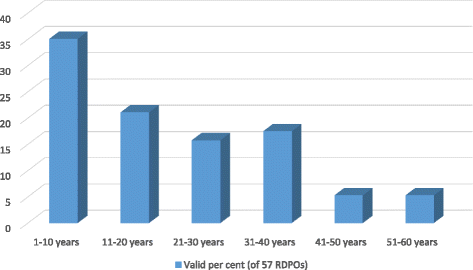
Methods: We reviewed 112 RDPO websites, conducted an online survey completed by 61 organisational leaders, and interviewed ten leaders and two key informants. Quantitative and qualitative data were analysed using basic descriptive statistics and content analysis, respectively.
Results: Although most are small volunteer-based groups, more than 90% of the surveyed RDPOs had a goal to promote or support research on the diseases affecting their members. Nearly all (95 %) had undertaken at least one research-related activity - such as providing funding or other support to researchers - in the previous five years. However, RDPO leaders reported considerable challenges in meeting their research goals. Difficulties most frequently identified were insufficient RDPO resources, and a perceived lack of researchers interested in studying their diseases. Other concerns included inadequate RDPO expertise in governing research "investments", and difficulty engaging researchers in the organisation's knowledge and ideas. We discuss these perceived challenges in the light of two systemic issues: the proliferation of and lack of collaboration between RDPOs, and the lack of specific governmental policies and resources supporting rare disease research and patient advocacy in Australia.
Conclusion: This study provides unique information about the experiences of RDPOs generally, rather than experiences retrospectively reported by RDPOs associated with successful research. We describe RDPOs' valuable contributions to research, while also providing insights into the difficulties for small organisations trying to promote research. The study is relevant internationally because of what it tells us about RDPOs; however, we draw attention to specific opportunities in Australia to support RDPOs' involvement in research, for the benefit of current and future generations affected by rare diseases.
Figures
Similar articles
-
'Advocacy groups are the connectors': Experiences and contributions of rare disease patient organization leaders in advanced neurotherapeutics.Health Expect. 2022 Dec;25(6):3175-3191. doi: 10.1111/hex.13625. Epub 2022 Oct 28. Health Expect. 2022. PMID: 36307981 Free PMC article.
-
The involvement of rare disease patient organisations in therapeutic innovation across rare paediatric neurological conditions: a narrative review.Orphanet J Rare Dis. 2022 Apr 18;17(1):167. doi: 10.1186/s13023-022-02317-6. Orphanet J Rare Dis. 2022. PMID: 35436886 Free PMC article. Review.
-
Beyond the black stump: rapid reviews of health research issues affecting regional, rural and remote Australia.Med J Aust. 2020 Dec;213 Suppl 11:S3-S32.e1. doi: 10.5694/mja2.50881. Med J Aust. 2020. PMID: 33314144
-
A snapshot of consumer engagement in clinical trials in Australia: results of a national survey of clinical trial networks and research organisations.Res Involv Engagem. 2022 Feb 5;8(1):3. doi: 10.1186/s40900-022-00338-w. Res Involv Engagem. 2022. PMID: 35123586 Free PMC article.
-
Equity consideration in palliative care policies, programs, and evaluation: an analysis of selected federal and South Australian documents.BMC Palliat Care. 2022 Jun 16;21(1):109. doi: 10.1186/s12904-022-00997-2. BMC Palliat Care. 2022. PMID: 35710402 Free PMC article. Review.
Cited by
-
Patient involvement in medical research: what patients and physicians learn from each other.Orphanet J Rare Dis. 2019 Jan 24;14(1):21. doi: 10.1186/s13023-018-0969-1. Orphanet J Rare Dis. 2019. PMID: 30678705 Free PMC article.
-
'Advocacy groups are the connectors': Experiences and contributions of rare disease patient organization leaders in advanced neurotherapeutics.Health Expect. 2022 Dec;25(6):3175-3191. doi: 10.1111/hex.13625. Epub 2022 Oct 28. Health Expect. 2022. PMID: 36307981 Free PMC article.
-
To what degree are orphan drugs patient-centered? A review of the current state of clinical research in rare diseases.Orphanet J Rare Dis. 2020 Jun 3;15(1):134. doi: 10.1186/s13023-020-01400-0. Orphanet J Rare Dis. 2020. PMID: 32493385 Free PMC article. Review.
-
The involvement of rare disease patient organisations in therapeutic innovation across rare paediatric neurological conditions: a narrative review.Orphanet J Rare Dis. 2022 Apr 18;17(1):167. doi: 10.1186/s13023-022-02317-6. Orphanet J Rare Dis. 2022. PMID: 35436886 Free PMC article. Review.
-
Emerging roles and opportunities for rare disease patient advocacy groups.Ther Adv Rare Dis. 2023 Apr 24;4:26330040231164425. doi: 10.1177/26330040231164425. eCollection 2023 Jan-Dec. Ther Adv Rare Dis. 2023. PMID: 37197559 Free PMC article.
References
-
- Orphanet. Orphanet website Australia entry point. [Internet] 2013 [cited 2014 29 Dec]; Available from: http://www.orpha.net/national/AU-EN/index/about-rare-diseases/.
-
- European Organisation for Rare Diseases. What is a rare disease? [Internet]. 2013 [cited 2013 Mar 4]; Available from: http://www.eurordis.org/content/what-rare-disease.
-
- Australian Bureau of Statistics [Internet]. 2014 [cited 2014 Dec 29]; Available from: www.abs.gov.au.
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical