Cardiovascular Health in Anxiety or Mood Problems Study (CHAMPS): study protocol for a randomized controlled trial
- PMID: 26754447
- PMCID: PMC4707770
- DOI: 10.1186/s13063-015-1109-z
Cardiovascular Health in Anxiety or Mood Problems Study (CHAMPS): study protocol for a randomized controlled trial
Abstract
Background: Previous psychological and pharmacological interventions have primarily focused on depression disorders in populations with cardiovascular diseases (CVDs) and the efficacy of anxiety disorder interventions is only more recently being explored. Transdiagnostic interventions address common emotional processes and the full range of anxiety and depression disorders often observed in populations with CVDs. The aim of CHAMPS is to evaluate the feasibility of a unified protocol (UP) for the transdiagnostic treatment of emotional disorders intervention in patients recently hospitalized for CVDs. The current study reports the protocol of a feasibility randomized controlled trial to inform a future trial.
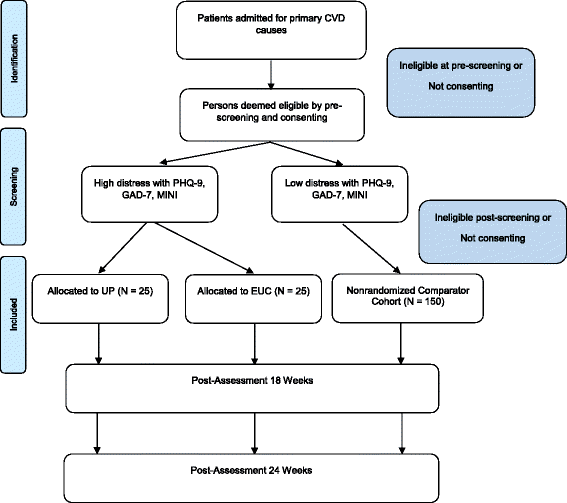
Methods/design: This is a feasibility randomized, controlled trial with a single-center design. A total of 50 participants will be block-randomized to either a UP intervention or enhanced usual care. Both groups will receive standard CVD care. The UP intervention consists of 1) enhancing motivation, readiness for change, and treatment engagement; (2) psychoeducation about emotions; (3) increasing present focused emotion awareness; (4) increasing cognitive flexibility; (5) identifying and preventing patterns of emotion avoidance and maladaptive emotion-driven behaviors (EDBs, including tobacco smoking, and alcohol use); (6) increasing tolerance of emotion-related physical sensations; (7) interoceptive and situation-based emotion-focused exposure; and (8) relapse prevention strategies. Treatment duration is 12 to 18 weeks. Relevant outcomes include the standard deviation of self-rated anxiety, depression and quality of life symptoms. Other outcomes include intervention acceptability, satisfaction with care, rates of EDBs, patient adherence, physical activity, cardiac and psychiatric readmissions. Parallel to the main trial, a nonrandomized comparator cohort will be recruited comprising 150 persons scoring below the predetermined depression and anxiety severity thresholds.
Discussion: CHAMPS is designed to evaluate the UP for the transdiagnostic treatment of emotional disorders targeting emotional disorder processes in a CVD population. The design will provide preliminary evidence of feasibility, attrition, and satisfaction with treatment to design a definitive trial. If the trial is feasible, it opens up the possibility for interventions to target broader emotional processes in the precarious population with CVD and emotional distress.
Trial registration: ACTRN12615000555550 , registered on 29/05/2015.
Figures
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

