Discrimination of cirrhotic nodules, dysplastic lesions and hepatocellular carcinoma by their vibrational signature
- PMID: 26754490
- PMCID: PMC4710034
- DOI: 10.1186/s12967-016-0763-6
Discrimination of cirrhotic nodules, dysplastic lesions and hepatocellular carcinoma by their vibrational signature
Abstract
Background: Hepatocarcinogenesis is a multistep process characterized in patients with chronic liver diseases by a spectrum of hepatic nodules that mark the progression from regenerative nodules to dysplastic lesions followed by hepatocellular carcinoma (HCC). The differential diagnosis between precancerous dysplastic nodules and early HCC still represents a challenge for both radiologists and pathologists. We addressed the potential of Fourier transform-infrared (FTIR) microspectroscopy for grading cirrhotic nodules on frozen tissue sections.
Methods: The study was focused on 39 surgical specimens including normal livers (n = 11), dysplastic nodules (n = 6), early HCC (n = 1), progressed HCC on alcoholic cirrhosis (n = 10) or hepatitis C virus cirrhosis (n = 11). The use of the bright infrared source emitted by the synchrotron radiation allowed investigating the biochemical composition at the cellular level. Chemical mapping on whole tissue sections was further performed using a FTIR microscope equipped with a laboratory-based infrared source. The variance was addressed by principal component analysis.
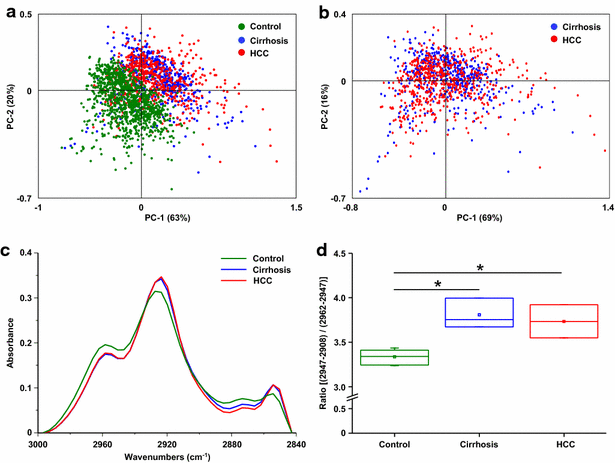
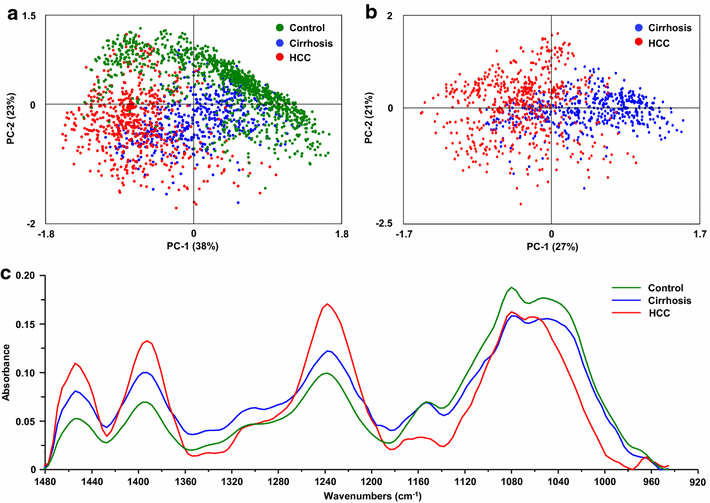
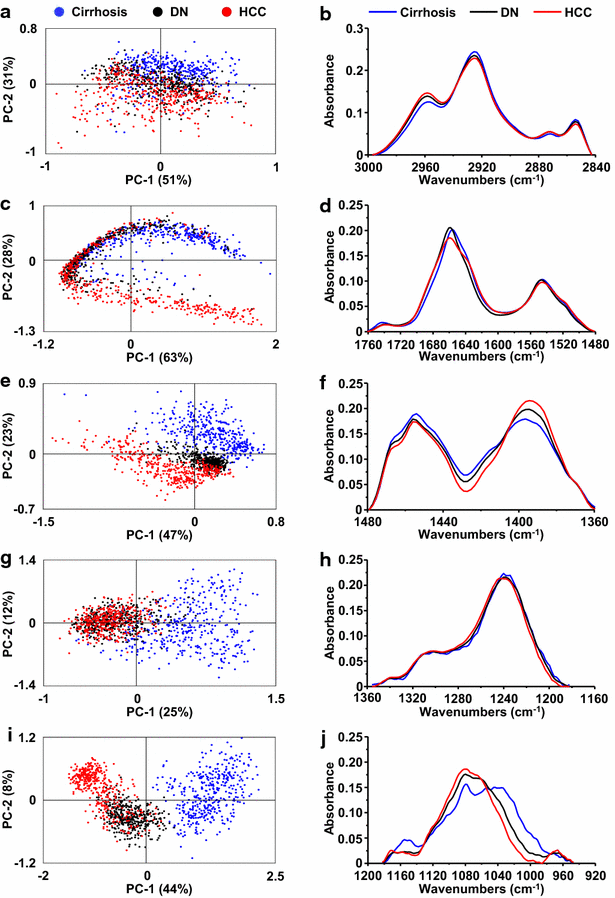
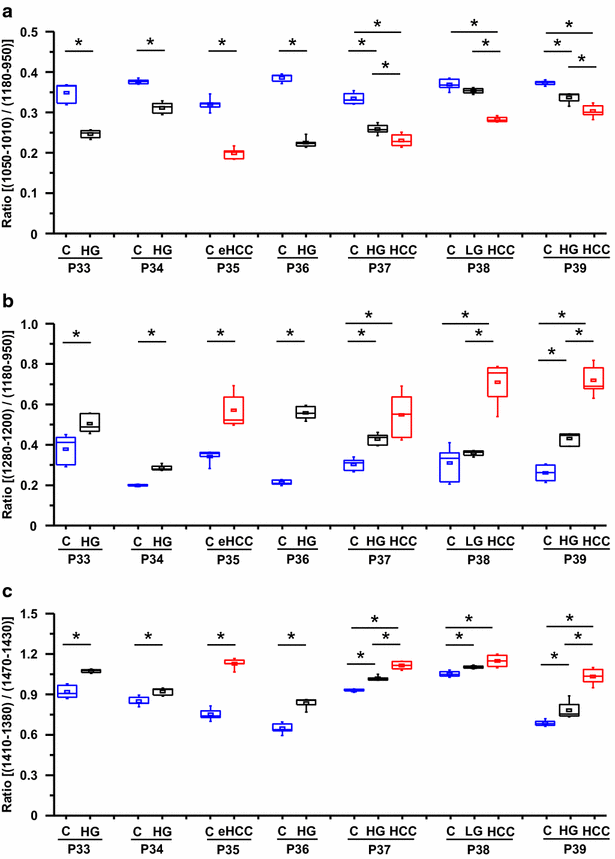
Results: Profound alterations of the biochemical composition of the pathological liver were demonstrated by FTIR microspectroscopy. Indeed, dramatic changes were observed in lipids, proteins and sugars highlighting the metabolic reprogramming in carcinogenesis. Quantifiable spectral markers were characterized by calculating ratios of areas under specific bands along the infrared spectrum. These markers allowed the discrimination of cirrhotic nodules, dysplastic lesions and HCC. Finally, the spectral markers can be measured using a laboratory FTIR microscope that may be easily implemented at the hospital.
Conclusion: Metabolic reprogramming in liver carcinogenesis can constitute a signature easily detectable using FTIR microspectroscopy for the diagnosis of precancerous and cancerous lesions.
Figures





Similar articles
-
In situ chemical composition analysis of cirrhosis by combining synchrotron fourier transform infrared and synchrotron X-ray fluorescence microspectroscopies on the same tissue section.Anal Chem. 2012 Dec 4;84(23):10260-6. doi: 10.1021/ac302072t. Epub 2012 Nov 13. Anal Chem. 2012. PMID: 23121424
-
A molecular signature to discriminate dysplastic nodules from early hepatocellular carcinoma in HCV cirrhosis.Gastroenterology. 2006 Dec;131(6):1758-67. doi: 10.1053/j.gastro.2006.09.014. Epub 2006 Sep 19. Gastroenterology. 2006. PMID: 17087938
-
Hepatocellular dysplastic nodules.Ann Ital Chir. 2008 Mar-Apr;79(2):81-9. Ann Ital Chir. 2008. PMID: 18727269
-
Hepatocarcinogenesis in liver cirrhosis: imaging diagnosis.J Korean Med Sci. 1998 Apr;13(2):103-16. doi: 10.3346/jkms.1998.13.2.103. J Korean Med Sci. 1998. PMID: 9610609 Free PMC article. Review.
-
Diagnosing Borderline Hepatic Nodules in Hepatocarcinogenesis: Imaging Performance.AJR Am J Roentgenol. 2015 Jul;205(1):10-21. doi: 10.2214/AJR.14.12655. AJR Am J Roentgenol. 2015. PMID: 26102378 Review.
Cited by
-
Advancements in medical research: Exploring Fourier Transform Infrared (FTIR) spectroscopy for tissue, cell, and hair sample analysis.Skin Res Technol. 2024 Jun;30(6):e13733. doi: 10.1111/srt.13733. Skin Res Technol. 2024. PMID: 38887131 Free PMC article. Review.
-
Identifying Apoptosis-Related Transcriptomic Aberrations and Revealing Clinical Relevance as Diagnostic and Prognostic Biomarker in Hepatocellular Carcinoma.Front Oncol. 2021 Feb 18;10:519180. doi: 10.3389/fonc.2020.519180. eCollection 2020. Front Oncol. 2021. PMID: 33680905 Free PMC article.
-
Point-of-care detection of fibrosis in liver transplant surgery using near-infrared spectroscopy and machine learning.Health Sci Rep. 2023 Oct 31;6(11):e1652. doi: 10.1002/hsr2.1652. eCollection 2023 Nov. Health Sci Rep. 2023. PMID: 37920655 Free PMC article.
References
-
- Kim SR, Ikawa H, Ando K, Mita K, Fuki S, Sakamoto M, et al. Multistep hepatocarcinogenesis from a dysplastic nodule to well-differentiated hepatocellular carcinoma in a patient with alcohol-related liver cirrhosis. World J Gastroenterol. 2007;13:1271–1274. doi: 10.3748/wjg.v13.i8.1271. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

