Electrical Stimulation to Enhance Axon Regeneration After Peripheral Nerve Injuries in Animal Models and Humans
- PMID: 26754579
- PMCID: PMC4824030
- DOI: 10.1007/s13311-015-0415-1
Electrical Stimulation to Enhance Axon Regeneration After Peripheral Nerve Injuries in Animal Models and Humans
Abstract
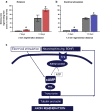
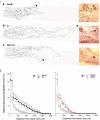
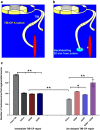
Injured peripheral nerves regenerate their lost axons but functional recovery in humans is frequently disappointing. This is so particularly when injuries require regeneration over long distances and/or over long time periods. Fat replacement of chronically denervated muscles, a commonly accepted explanation, does not account for poor functional recovery. Rather, the basis for the poor nerve regeneration is the transient expression of growth-associated genes that accounts for declining regenerative capacity of neurons and the regenerative support of Schwann cells over time. Brief low-frequency electrical stimulation accelerates motor and sensory axon outgrowth across injury sites that, even after delayed surgical repair of injured nerves in animal models and patients, enhances nerve regeneration and target reinnervation. The stimulation elevates neuronal cyclic adenosine monophosphate and, in turn, the expression of neurotrophic factors and other growth-associated genes, including cytoskeletal proteins. Electrical stimulation of denervated muscles immediately after nerve transection and surgical repair also accelerates muscle reinnervation but, at this time, how the daily requirement of long-duration electrical pulses can be delivered to muscles remains a practical issue prior to translation to patients. Finally, the technique of inserting autologous nerve grafts that bridge between a donor nerve and an adjacent recipient denervated nerve stump significantly improves nerve regeneration after delayed nerve repair, the donor nerves sustaining the capacity of the denervated Schwann cells to support nerve regeneration. These reviewed methods to promote nerve regeneration and, in turn, to enhance functional recovery after nerve injury and surgical repair are sufficiently promising for early translation to the clinic.
Keywords: Delayed nerve repair; Electrical stimulation; Peripheral nerve injury; Peripheral nerve regeneration; Side-to-side crossbridges.
Conflict of interest statement
Compliance with Ethical Standards Required Author Forms Disclosure forms provided by the authors are available with the online version of this article.
Figures


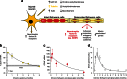
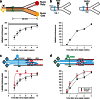
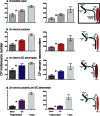
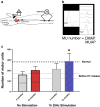
References
-
- Fenrich K, Gordon T. Canadian Association of Neuroscience review: axonal regeneration in the peripheral and central nervous systems-current issues and advances. Neurol Sci 2004;31:142--156. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

