Diacylglycerol lipase disinhibits VTA dopamine neurons during chronic nicotine exposure
- PMID: 26755579
- PMCID: PMC4743781
- DOI: 10.1073/pnas.1522672113
Diacylglycerol lipase disinhibits VTA dopamine neurons during chronic nicotine exposure
Abstract
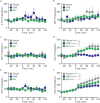
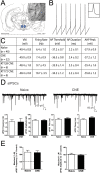
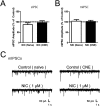
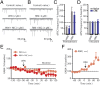
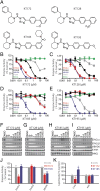
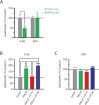
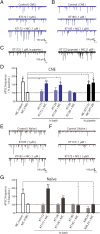
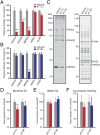
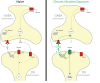
Chronic nicotine exposure (CNE) alters synaptic transmission in the ventral tegmental area (VTA) in a manner that enhances dopaminergic signaling and promotes nicotine use. The present experiments identify a correlation between enhanced production of the endogenous cannabinoid 2-arachidonoylglycerol (2-AG) and diminished release of the inhibitory neurotransmitter GABA in the VTA following CNE. To study the functional role of on-demand 2-AG signaling in GABAergic synapses, we used 1,2,3-triazole urea compounds to selectively inhibit 2-AG biosynthesis by diacylglycerol lipase (DAGL). The potency and selectivity of these inhibitors were established in rats in vitro (rat brain proteome), ex vivo (brain slices), and in vivo (intracerebroventricular administration) using activity-based protein profiling and targeted metabolomics analyses. Inhibition of DAGL (2-AG biosynthesis) rescues nicotine-induced VTA GABA signaling following CNE. Conversely, enhancement of 2-AG signaling in naïve rats by inhibiting 2-AG degradation recapitulates the loss of nicotine-induced GABA signaling evident following CNE. DAGL inhibition reduces nicotine self-administration without disrupting operant responding for a nondrug reinforcer or motor activity. Collectively, these findings provide a detailed characterization of selective inhibitors of rat brain DAGL and demonstrate that excessive 2-AG signaling contributes to a loss of inhibitory GABAergic constraint of VTA excitability following CNE.
Keywords: 2-arachidonoylglycerol; GABA; diacylglycerol lipase; nicotine; ventral tegmental area.
Conflict of interest statement
Conflict of interest statement: B.F.C. is a cofounder and advisor for a biotechnology company interested in developing inhibitors of serine hydrolases as therapeutic targets.
Figures





References
-
- Mechoulam R, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50(1):83–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 AA006420/AA/NIAAA NIH HHS/United States
- U01 AA013498/AA/NIAAA NIH HHS/United States
- U54 MH084512/MH/NIMH NIH HHS/United States
- F32 AA020430/AA/NIAAA NIH HHS/United States
- K99 DA035865/DA/NIDA NIH HHS/United States
- R01 DA033760/DA/NIDA NIH HHS/United States
- F32 DA029994/DA/NIDA NIH HHS/United States
- R01 AA013498/AA/NIAAA NIH HHS/United States
- P60 AA006420/AA/NIAAA NIH HHS/United States
- K99 DA035864/DA/NIDA NIH HHS/United States
- R01 DA009789/DA/NIDA NIH HHS/United States
- R01AA020404/AA/NIAAA NIH HHS/United States
- R37 AA017447/AA/NIAAA NIH HHS/United States
- R01 AA020404/AA/NIAAA NIH HHS/United States
- R01 MH084512/MH/NIMH NIH HHS/United States
- P01 DA009789/DA/NIDA NIH HHS/United States
- R01 AA015566/AA/NIAAA NIH HHS/United States
- R00 DA035864/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

